Compound bone cement with function of reducing thermal necrosis effect
A bone cement and low-heat technology, applied in the field of materials, can solve the problems of inability to form external callus, PMMA thermal necrosis, poor mechanical strength, etc., and achieve the effects of reducing thermal necrosis, lowering the maximum temperature, and maintaining the curing rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation and characterization of PMMA-paraffin / silica (PMMA10) bone cement
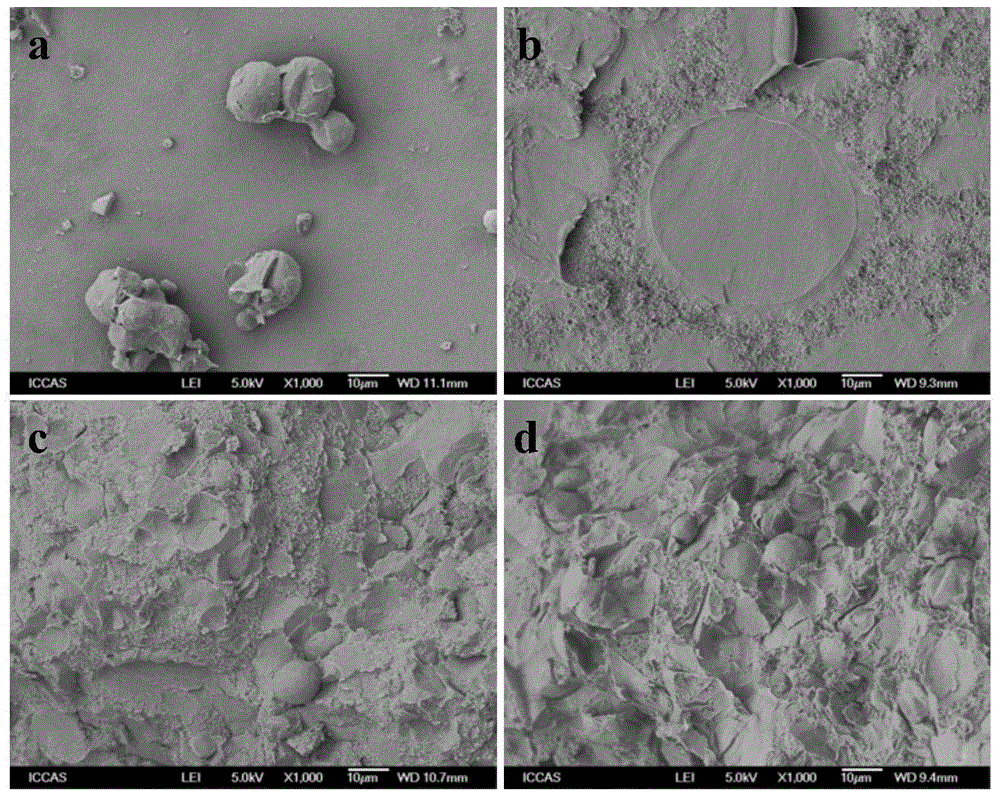
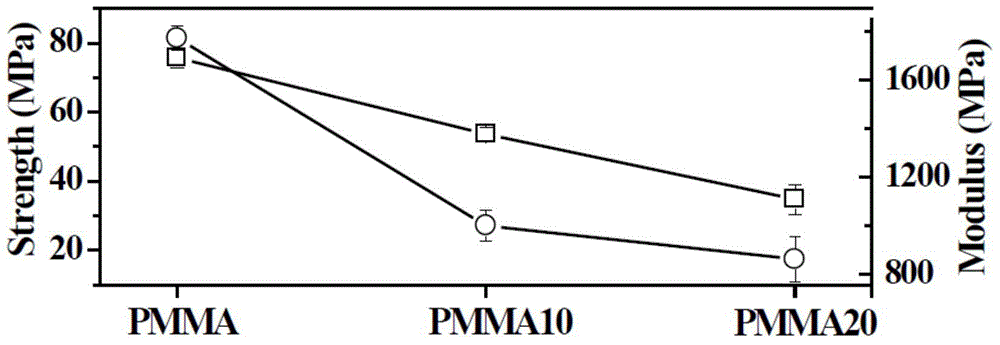
[0035]Mix 9g of currently clinically used PMMA powder (MENDECSPINE, the main components are polymethylmethacrylate 67.5wt%, barium sulfate 30wt%, and diphenyl peroxide 2.5wt%) with 1g paraffin / silica microcapsules Put it into a ball mill jar and mix at low speed for 30 minutes to obtain the solid phase components. Weigh 8g of the solid phase component and put it into a 20ml syringe, add the liquid phase component (the mass fraction of methyl methacrylate is 99.1%, the mass fraction of DMPT is 0.9%, and hydroquinone is 75ppm) to control the solid-liquid ratio 2:1 (m / v), fully stirred for 1 min, injected into the mold, and cured to obtain PMMA10 composite bone cement. Its surface morphology is as figure 1 As shown in c, it can be seen from the figure that the paraffin / silica microcapsule structure is stable and can be tightly combined with PMMA. The maximum exothermic temperature ...
Embodiment 2
[0036] Example 2 Preparation and characterization of PMMA-paraffin / silica (PMMA20) bone cement
[0037] Mix 8 g of currently clinically used PMMA powder (MENDECSPINE) and 2 g of paraffin / silicon dioxide microcapsules into a ball mill, and mix at a low speed for 30 minutes to obtain a solid phase component. Weigh 8g of the solid phase component and put it into a 20ml syringe, add the liquid phase component (the mass fraction of methyl methacrylate is 99.1%, the mass fraction of DMPT is 0.9%, and hydroquinone is 75ppm) to control the solid-liquid ratio 2:1 (m / v), fully stirred for 1 min, injected into the mold, and cured to obtain PMMA20 composite bone cement. Its surface morphology is as figure 1 As shown in d, it can be seen from the figure that the surface of the material becomes rougher, but the paraffin / silica microcapsule structure is stable and can be tightly combined with PMMA. The maximum exothermic temperature of PMMA20 bone cement during curing is 44°C, which is les...
Embodiment 3
[0038] Example 3 Preparation and characterization of PMMA-paraffin / silica (PMMA30) bone cement
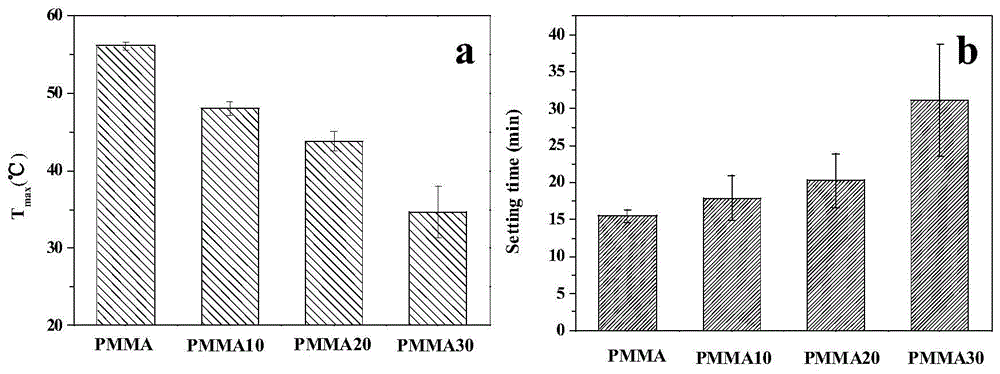
[0039] Mix 7 g of currently clinically used PMMA powder (MENDECSPINE) and 3 g of paraffin / silicon dioxide microcapsules into a ball mill, and mix at a low speed for 30 minutes to obtain a solid phase component. Weigh 8g of the solid phase component and put it into a 20ml syringe, add 4ml of the liquid phase component (the mass fraction of methyl methacrylate is 99.1%, the mass fraction of DMPT is 0.9%, and hydroquinone is 75ppm) to control the solid-liquid The ratio is 1.5:1 (m / v), fully stirred for 1 min, injected into the mold, and cured to obtain PMMA30 composite bone cement. The maximum exothermic temperature during the curing process of PMMA30 bone cement was 35°C ( figure 2 a), less than the normal body temperature of the human body; curing time is 32min ( figure 2 b), the feasibility of clinical operation is poor.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com