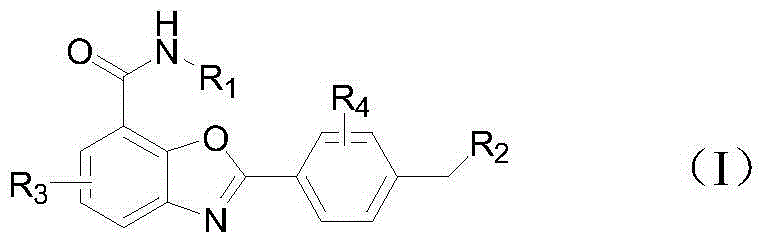
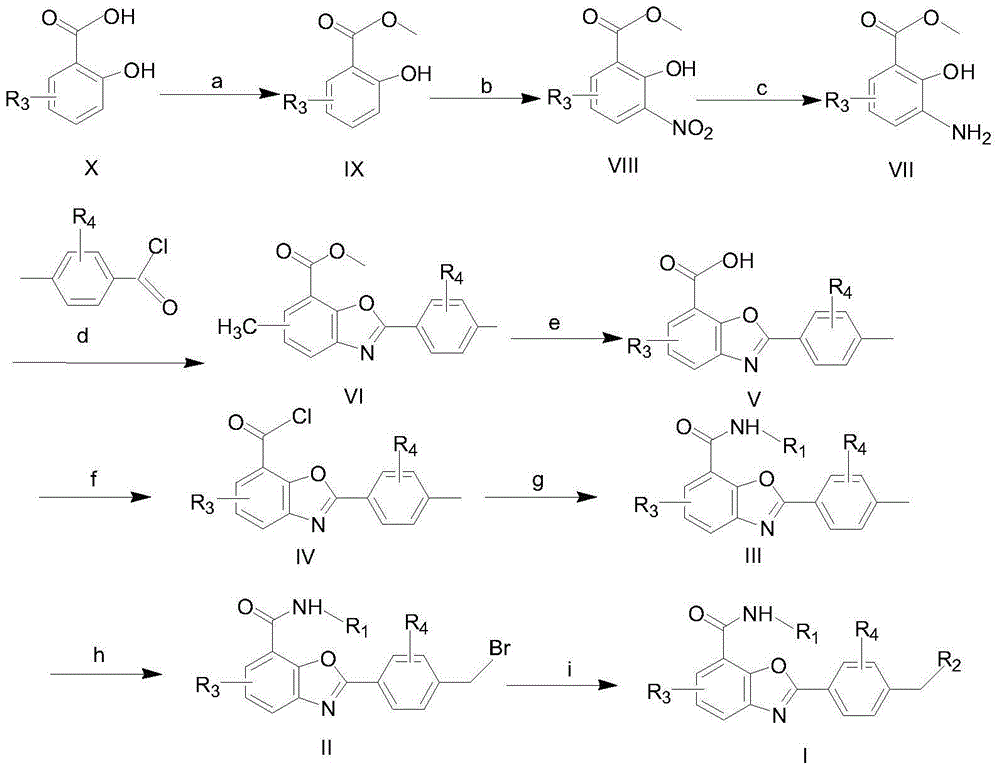
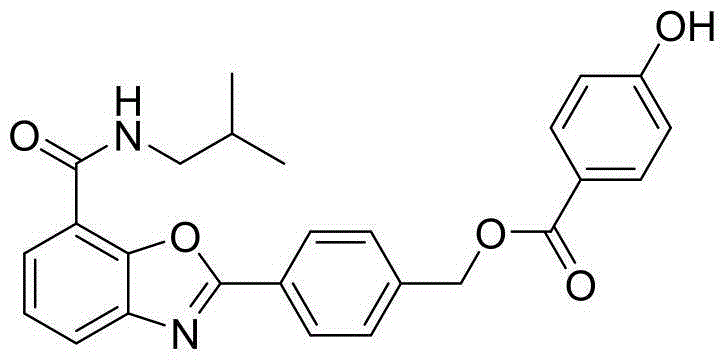
Benzoxazole compound and preparation method and application thereof
A technology for benzoxazoles and compounds, which is applied in the field of benzoxazoles and their preparation, can solve the problems that surface toxic proteins cannot be anchored, the infectivity of pathogenic bacteria is decreased, and the like, and achieve the effect of inhibiting the infection of Staphylococcus aureus.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] This embodiment relates to the preparation of methyl 2-hydroxybenzoate (IX)
[0042] Add 100mL of methanol to 10g of salicylic acid, control the temperature in the ice-salt bath at 0°C, slowly add 10mL of thionyl chloride dropwise, control the temperature at 0°C during the dropwise addition, and raise the temperature to 75°C for reflux reaction after the dropwise addition, after 16h , the reaction solution was cooled to room temperature, the reaction solution was concentrated, dissolved by adding 200mL ethyl acetate, and successively added 100mL saturated sodium bicarbonate solution, 100mL water and 100mL saturated brine to wash the organic layer, added anhydrous sodium sulfate to dry, concentrated the organic layer, 10.3 g of holly liquid was obtained, yield 94.5%.
Embodiment 2
[0044] Preparation of 3-nitro-2-hydroxybenzoic acid methyl ester (VIII)
[0045] Dissolve 10g of methyl 2-hydroxybenzoate and 7mL of 65% nitric acid in 150mL of dichloromethane, control the temperature in the ice-salt bath at 0°C, slowly add 10mL of concentrated sulfuric acid dropwise, control the temperature in the ice-salt bath at 0°C, and end the addition Finally, the reaction temperature was still controlled at 0°C. After 20 min, 150 mL of water was added to quench the reaction, followed by adding 150 mL of saturated sodium bicarbonate solution, 150 mL of water, and 150 mL of saturated brine to wash the organic layer, adding anhydrous sodium sulfate to dry, and concentrating the organic layer , separated by column chromatography with PE:EA=6:1 to obtain 3.8g of yellow solid with a yield of 29.3%.
Embodiment 3
[0047] Preparation of 3-amino-2-hydroxybenzoic acid methyl ester (VII)
[0048] Dissolve 3.5g of methyl 3-nitro-2-hydroxybenzoate and 350mg of palladium on carbon in 50mL of methanol, and react at room temperature under reflux in a hydrogen system. After 18 hours, filter, add anhydrous sodium sulfate to the filtrate to dry, and concentrate the reaction solution. 2.4 g of white solid was obtained by column chromatography with PE:EA=8:1, the yield was 82.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com