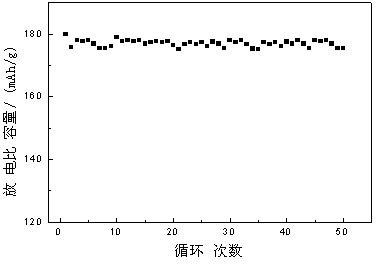
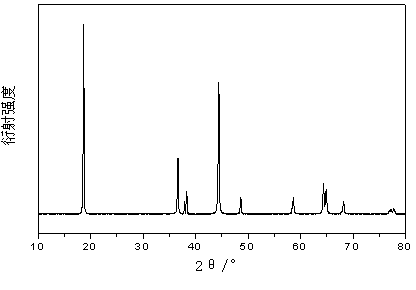
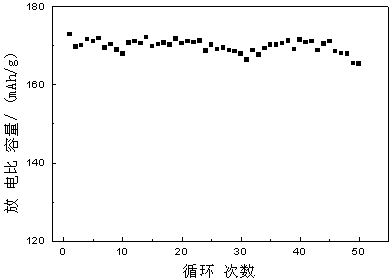
Preparation method of high-capacity lithium nickel cobalt aluminate
A lithium nickel cobalt aluminate, high-capacity technology, applied in the field of lithium ion batteries, can solve the problems of poor electrochemical performance of materials, uneven particle size, difficult process control, etc. Excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Lithium carbonate, nickelous oxide, cobalt oxide, and aluminum oxide are weighed according to the molar ratio Li: Ni: Co: Al=1:0.8:0.15:0.05 and added to the ball mill tank with a ball-to-material ratio of 8 :1. The mixed raw materials were mixed by ball milling at room temperature for 10 hours, and then under the state of ball milling, the temperature was raised to 500°C at a speed of 10°C / min under the condition of oxygen flow, and ball milled at constant temperature for 5 hours, and then under the state of ball milling, under the condition of oxygen flow The temperature was raised to 750°C at a speed of 8°C / min, and ball milled at this temperature for 15 hours at a constant temperature. After sintering, it was cooled to a lower temperature in the state of ball milling and then released from the furnace to obtain the powder positive electrode material nickel cobalt lithium aluminate. The ball milling speed was 200r / min during the whole sintering process.
...
Embodiment 2
[0023] Example 2: Lithium hydroxide, nickel oxide, cobalt oxide, and aluminum hydroxide were weighed in a molar ratio of Li:Ni:Co:Al=1:0.62:0.3:0.08 and added to the ball mill tank with a ball-to-material ratio of 12. :1. The mixed raw materials were mixed by ball milling at room temperature for 5 hours, then under the state of ball milling, the temperature was raised to 400°C at a speed of 12°C / min under the condition of oxygen flow, and ball milled at constant temperature for 6 hours, and then under the state of ball milling, under the condition of air flow The temperature was raised to 800°C at a speed of 10°C / min, and ball milled at this temperature for 20 hours at a constant temperature. After sintering, the powder was cooled to a lower temperature in the state of ball milling and then released from the furnace to obtain the powder positive electrode material nickel cobalt lithium aluminate. The ball milling speed was 150r / min during the whole sintering process.
[0024]...
Embodiment 3
[0025] Example 3: Lithium oxalate, nickel carbonate, cobalt nitrate, and aluminum nitrate were weighed into the ball mill tank according to the molar ratio Li:Ni:Co:Al=1:0.8:0.1:0.1, and the ball-to-material ratio was 15:1. The mixed raw materials were mixed by ball milling at room temperature for 20 hours, and then in the state of ball milling, the temperature was raised to 600°C at a speed of 20°C / min under the condition of air flow, and ball milled at a constant temperature for 10 hours, and then in the state of ball milling, under the condition of air flow, the temperature was The temperature was raised to 900°C at a rate of 15°C / min, and ball milled at a constant temperature for 30 hours at this temperature. After sintering, cooled to a lower temperature in the state of ball milling and then released from the furnace to obtain the powder positive electrode material nickel cobalt lithium aluminate. The ball milling speed was 600r / min during the whole sintering process.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com