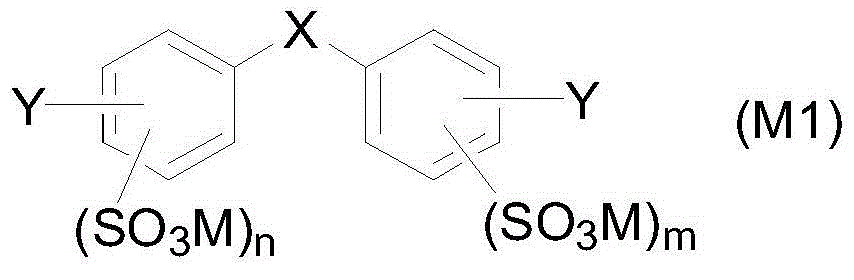
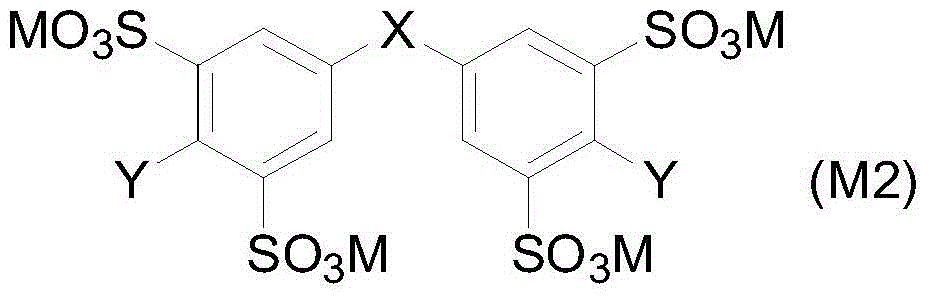
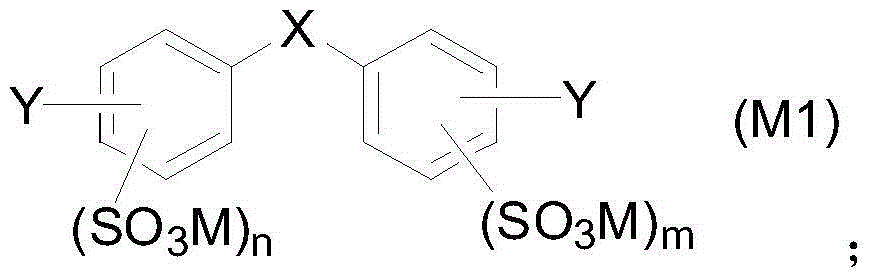Aryl sulfonic acid compound purifying method
A technology of aryl sulfonic acid and purification method, which is applied in the field of purification of aryl sulfonic acid compounds, can solve problems such as side reactions and limit the types of aryl sulfonic acid salts, and achieve the effect of reducing purification costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Take 2.0L of the diluted solution after the sulfonation reaction (4,4'-difluoro-3,3'5,5'-tetrasulfonic acid benzophenone compound has a mass of about 186g and sulfuric acid is about 148g in aqueous solution), Gradually add Ca(OH) under the condition of full stirring 2 Suspension (a mixture of 180.0g calcium hydroxide and 150mL deionized water), control the temperature below 45°C. After the addition was completed, the filtrate was filtered, washed and combined to obtain a pink clear liquid. Under the conditions indicated by the pH meter, add an aqueous solution containing 10.0 g of calcium hydroxide to pH = 6.50, filter with suction, wash, and combine to obtain about 2.5 L of light yellow liquid. Add 90.0 g of oxalic acid dihydrate to the solution, control the temperature at 50° C., react for 3 h, filter with suction, wash and combine. Take a small amount of clear liquid and titrate it with the above-mentioned saturated sodium carbonate solution to confirm that the aci...
Embodiment 2
[0028] Take 2.0L of the diluted solution after the sulfonation reaction (4,4'-difluoro-3,3'5,5'-tetrasulfonic acid benzophenone compound has a mass of about 186g and sulfuric acid is about 148g in aqueous solution), Gradually add CaCO under full stirring 3 Suspension (a mixture of 233.2g calcium carbonate and 250mL deionized water), control the temperature below 45°C. After the addition was completed, the filtrate was filtered, washed and combined to obtain a pink clear liquid. Under the conditions indicated by the pH meter, add an aqueous solution containing 15.0 g of calcium carbonate to pH = 7.50, filter with suction, wash, and combine to obtain about 2.5 L of light yellow liquid. Add 65.0 g of anhydrous oxalic acid to the solution, control the temperature at 50°C, react for 3 hours, filter with suction, wash and combine. Take a small amount of clear liquid and titrate it with the above-mentioned saturated sodium carbonate solution to confirm that the acidification has be...
Embodiment 3
[0030] Take 2.0L of the diluted solution after the sulfonation reaction (4,4'-difluoro-3,3'5,5'-tetrasulfonic acid benzophenone compound has a mass of about 186g and sulfuric acid is about 148g in aqueous solution), Gradually add Ca(OH) under the condition of full stirring 2 Suspension (a mixture of 180.0g calcium hydroxide and 150mL deionized water), control the temperature below 45°C. After the addition was completed, the filtrate was filtered, washed and combined to obtain a pink clear liquid. Under the conditions indicated by the pH meter, add an aqueous solution containing 15.0 g of calcium hydroxide to pH = 9.50, filter with suction, wash, and combine to obtain about 2.5 L of light yellow liquid. Add 90.0 g of oxalic acid dihydrate to the solution, control the temperature at 50° C., react for 3 h, filter with suction, wash and combine. Take a small amount of clear liquid and titrate it with the above-mentioned saturated sodium carbonate solution to confirm that the aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com