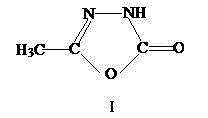
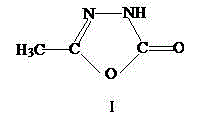
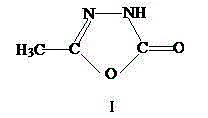
Method for synthesizing pymetrozine intermediate (oxadiazole ketone) by utilizing halogenated formate ester
A technology of haloformic acid ester and oxadiazolone, which is applied in the field of preparation of oxadiazolone, an intermediate of high-efficiency insecticide pymetrozine, can solve the problems of tail gas degradation failure, mass casualties, and highly toxic phosgene to reduce major safety hazards, the reaction conditions are mild and easy to control, and the process is safe and reliable.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]In the oxadiazolone 500ml reaction bottle, install agitator, thermometer, constant pressure dropping funnel and condenser, and all instruments will be dry and anhydrous. At room temperature, add 300ml of solvent 1,2-dichloroethane, 37.0g of acetylhydrazine, 46.5g of acid-binding agent sodium bicarbonate, start stirring, cool down, control the reaction temperature to about -10°C, and slowly add 52.0g of chloroformic acid dropwise 100ml dichloroethane solution of methyl ester, keep warm for 1.5hr after feeding, heat slowly to 30°C-35°C for 0.5h after reaction is stable, slowly heat up to reflux, reflux keep warm for 6-7 hours, take samples Analysis, when the residual acetylhydrazine ≤ 1.0%, the reaction is basically over, and the temperature is properly lowered to 25-50 °C, and 20.0 g of the catalyst 30% sodium methoxide solution is added under a slight negative pressure, and the temperature is slowly raised to reflux for 6.0 hours. The continuous removal of product promot...
Embodiment 2
[0046] In the oxadiazolone 500ml reaction bottle, install agitator, thermometer, constant pressure dropping funnel and condenser, and all instruments will be dry and anhydrous. At room temperature, add 250ml of solvent toluene and 37.0g of acetylhydrazide, start stirring, cool down, control the reaction temperature from -15°C to 50°C, slowly add 50.0g of methyl chloroformate in 100ml of toluene solution dropwise, and keep warm for reaction after the addition is complete 4.0hr, after the reaction is stable, heat slowly to 30°C-60°C and keep it warm for 1.5 hours, then slowly heat up to reflux, keep it under reflux for 12 hours, take a sample for analysis, when the residual acetylhydrazine ≤ 1.0%, the reaction is basically over, and the temperature should be lowered appropriately Add catalyst 30% sodium methoxide solution 20.0-40.0g under slight negative pressure at 25-50°C, check the pH of the reaction solution, slowly raise the temperature to reflux and keep it warm for 8.0 hou...
Embodiment 3
[0048] In the oxadiazolone 1000ml reaction bottle, install agitator, thermometer, constant pressure dropping funnel and condenser, and all instruments will be dry and anhydrous. At room temperature, add 500ml of solvent chloroform, 74.0g of acetylhydrazide, 60.0g of acid-binding agent sodium carbonate and 27.5g of sodium methoxide, start stirring, cool down, control the reaction temperature -20°C-20°C, and slowly add 108.3g dropwise 200ml chloroform solution of methyl chloroformate, keep warm for 3.0hr after feeding, heat slowly to 25°C-35°C for 1.5 hours after the reaction is stable, slowly heat up to reflux, keep warm at reflux for 3 hours, take samples for analysis , when the residue of acetylhydrazine ≤ 1.0%, the reaction is basically over, filter and wash while it is hot to remove inorganic salts, collect the filtrate and transfer it to another 1000ml reaction bottle of the same device, stir, cool down to room temperature, and add 18.5g of catalyst phosphorus pentoxide an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com