Preparation method of double-longchain alkyl methyl carboxyl glycine betaine
A technology of long-chain alkylmethyl carboxylate and betaine, which is applied in the field of preparation of double long-chain alkylmethyl carboxybetaine, and can solve problems such as increased hydrolysis of chloroacetic acid or sodium chloroacetate, poor emulsification, waste of resources, etc. , to avoid hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
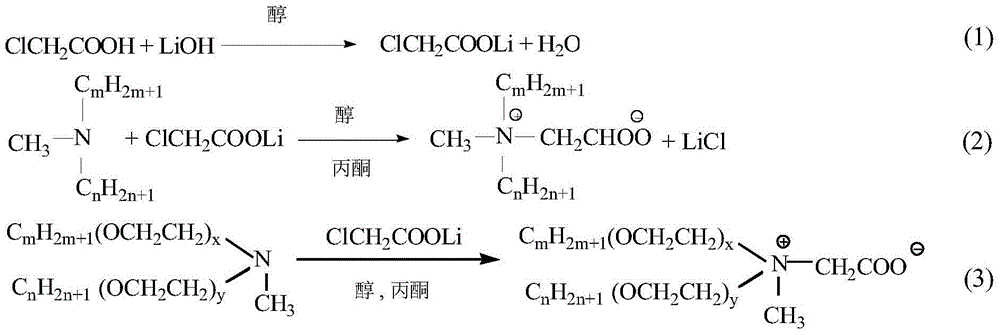
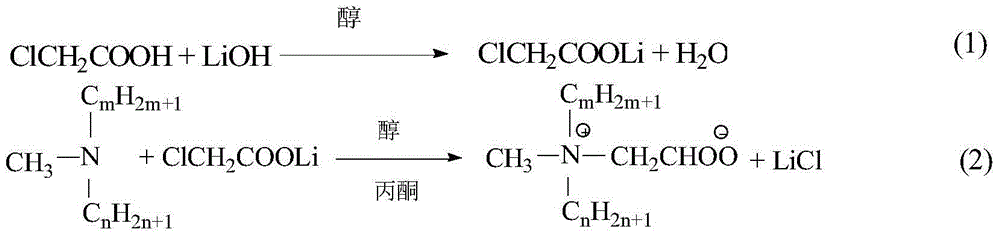
[0021] The preparation of embodiment 1 lithium chloroacetate ethanol solution
[0022] Add 50 mL of absolute ethanol and 4.87 g of chloroacetic acid (0.05 mol) into a 250 mL Erlenmeyer flask, shake gently until the chloroacetic acid is completely dissolved, then add 0.055 mol of lithium hydroxide (LiOH·H 2 O) Solid particles. Place the Erlenmeyer flask on a magnetic stirrer, and stir the reaction at room temperature for about 30 minutes until the LiOH solid particles disappear and the solution becomes clear. Add an appropriate amount of anhydrous Na to the Erlenmeyer flask 2 SO 4 , shake evenly to absorb the water generated in the reaction, let stand for 10 minutes and then suction filter, collect the filtrate to obtain lithium chloroacetate ethanol solution, the concentration of lithium chloroacetate is about 1mol / L.
Embodiment 2
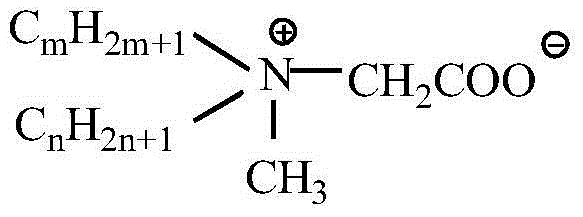
[0023] Embodiment 2 Didodecylmethylcarboxybetaine (diC 12 B) Preparation
[0024] Add 0.04mol (about 15g) didodecylmethyl tertiary amine (diC 12 A) and 0.06mol lithium chloroacetate (60mL ethanol solution), then add 60mL acetone and KI accounting for 3% of the total mass of the reaction system (including the solvent), put into a magnetic stirring bar, cover, and tighten the seal. Put it into an oil bath, control the temperature of the oil bath at 100±2°C, and continue the reaction for 20 hours at an appropriate rotation speed. After the reaction, cool down, remove the outer cover, take a sample, extract the unreacted tertiary amine in the product with petroleum ether under alkaline conditions, titrate with hydrochloric acid-isopropanol solution, and calculate the conversion rate of the tertiary amine. The conversion rate of tertiary amine reaches 91%.
Embodiment 3
[0025] Embodiment 3 Asymmetric double long-chain alkyl methyl carboxy betaine (C m+n B) Preparation
[0026]Add 0.04mol (about 15g) homemade unsymmetrical double long-chain alkylmethyl tertiary amine (C 14+10 A, C 16+8 A, C 18+6 A) and 0.06mol lithium chloroacetate (60mL ethanol solution), then add 60mL acetone and KI accounting for 3% of the total mass of the reaction system (including the solvent), put into a magnetic stirring bar, cover, and tighten the seal. Put it into an oil bath, control the temperature of the oil bath at 100±2°C, and continue the reaction for 20 hours at an appropriate rotation speed. After the reaction, cool down, remove the outer cover, take a sample, extract the unreacted tertiary amine in the product with petroleum ether under alkaline conditions, titrate with hydrochloric acid-isopropanol solution, and calculate the conversion rate of the tertiary amine. to C 14+10 A.C 16+8 A and C 18+6 A three kinds of unsymmetrical double long-chain alkyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com