A method for fermenting and transforming soybean isoflavone glycosides into aglycones from Grifola frondosa
A technology for isoflavone glycosides and Grifola frondosa, which is applied in the field of Grifola frondosa fermenting and transforming soybean isoflavone glycosides to generate aglycones, can solve the problems that the conversion efficiency of β-glucosidase needs to be improved, and achieves improved activity and absorption rate, no The effect of organic solvent residue and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
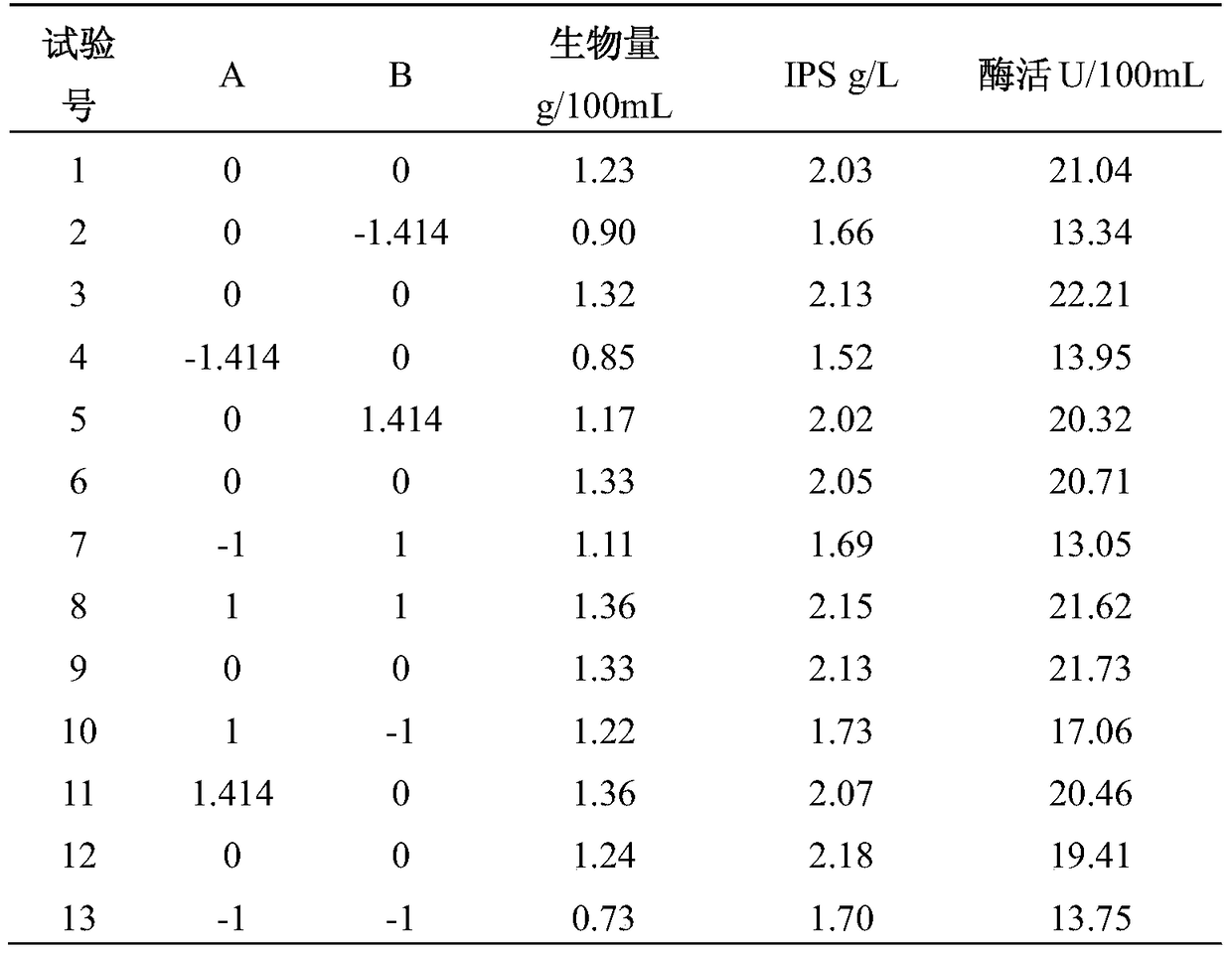
[0026] Embodiment 1 Grifola frondosa liquid fermentation produces β-glucosidase
[0027] 1. Method for measuring the activity of β-glucosidase
[0028] (1) Preparation of standard curve
[0029] Accurately weigh 13.90 mg of pNP (p-nitrophenol), dissolve it in distilled water and dilute to 100 mL. Take six 10mL volumetric flasks, draw 100, 200, 300, 400, 500, and 600 μL of pNP solution to bottles 1-6 respectively, and use 1mol / L Na 2 CO 3 The solution was made to volume and mixed evenly. The pNP concentrations corresponding to bottles 1-6 were 0.01, 0.02, 0.03, 0.04, 0.05, and 0.06 mmol / L. Measure the absorbance at 400 nm with distilled water as a control and draw a standard curve.
[0030] (2) Determination of β-glucosidase activity in the sample
[0031] Take 1 mL of the homogenized fermentation broth in a centrifuge tube, and centrifuge at 10,000 rpm for 10 min at 4°C. Mix 100 μL supernatant with 200 μL 5mM pNPG, incubate at 50°C for 30 min, add 2 mL 1mol / L Na 2 CO 3 ...
Embodiment 2
[0043] Example 2 β-glucosidase biotransformation of soybean isoflavone glycosides
[0044] 1. β-glucosidase biotransformation of soybean isoflavone glycosides to produce aglycones
[0045] Take 3-5 g of soybean isoflavone extract as a substrate, mix it with 100 mL of homogenized fermentation broth, and carry out a stirring reaction (transformation) at 40° C. to 60° C. for 12 to 20 hours to obtain soybean isoflavone aglycone. (optimization of the above parameters, as described in part 3 of this example)
[0046] 2. HPLC detection of soybean isoflavones
[0047] Liquid chromatography was used for detection, and the chromatographic column was C18, 4.6 mm×250 mm, with a particle size of 5 μm; the mobile phases A and B were acetonitrile and pH=3.0 phosphoric acid aqueous solution respectively, and the gradient elution conditions were shown in Table 3. The flow rate was 1.0 mL / min, the detection wavelength was 260 nm, the injection volume was 10 μL, and the column temperature was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com