High-capacity boron affinity separation material and preparation method and application thereof
A separation material, high capacity technology, applied in separation methods, chemical instruments and methods, preparation of samples for testing, etc. Unsatisfactory and other problems, to achieve good application prospects, improved sensitivity and accuracy, and high recovery rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
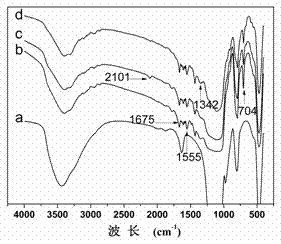
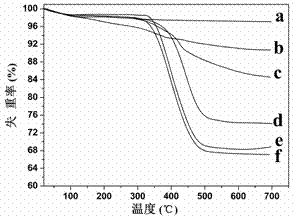
[0026] Example 1: Preparation of high-capacity boron affinity material based on silica gel
[0027] (1) Weigh 5.0 g of activated silica gel, then disperse it in 100 mL of redistilled toluene, 2.0 mL of 82.5 mM 4-chloromethylphenyltrimethoxysilane (4-CPTS), and react at 110°C After 12 h, the product was washed with toluene, methanol, and acetone for 3-4 times in sequence, and dried in vacuum at 50°C to obtain silica gel (silica-Cl for short) with surface-bonded initiator;
[0028] (2) Weigh 1.0 g of the above silica-Cl, ultrasonically disperse it in N,N-dimethylformamide, add 1.6 g of 3-acrylamidophenylboronic acid and 0.20 g of 2, 2'-bipyridine; the mixture is frozen repeatedly - Vacuum-thawing cycle, quickly add 0.10 g cuprous bromide under nitrogen protection; keep stirring, react at 90 °C for 8-16 hours, then wash with methanol and water in sequence. In order to thoroughly clean the residual catalyst, the obtained silica particles were dispersed in 60 mL methanol / 0.25 M ED...
Embodiment 2
[0033] Example 2: Preparation of high-capacity boron affinity separation material based on silica gel-coated magnetic nanoparticles
[0034] (1) Weigh 2.0 g of ferroferric oxide magnetic nanoparticles coated with silica gel by hydrolysis of ethyl orthosilicate, and then disperse them in 100 mL of double-distilled toluene, 2.0 mL of 120.2 mM 4-chloromethylbenzene Trimethoxysilane (4-CPTS) was reacted at 110°C for 12 h. After the reaction, the product was washed with toluene, methanol and acetone for 3-4 times, and dried in vacuum at 50°C to obtain magnetic nanoparticles with surface-bonded initiators. ;
[0035] (2) Weigh 1.0 g of the above-mentioned magnetic particles, ultrasonically disperse them in N,N-dimethylformamide, add 1.6 g of 3-acrylamidophenylboronic acid and 0.20 g of 2, 2'-bipyridyl; the mixture is repeatedly frozen- Vacuum-thawing cycle, quickly add 0.10 g cuprous bromide under nitrogen protection; keep stirring, react at 90 °C for a certain period of time, then...
Embodiment 3
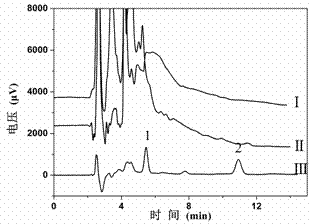
[0038] Example 3: Adsorption of catechol by a high-capacity boron affinity separation material based on silica gel
[0039] Weigh a series of 20 mg of the silica gel-based high-capacity boron affinity material prepared in Example 1, add 5 mL of catechol solutions of different concentrations, ultrasonically disperse, and shake at 25°C with a constant temperature oscillator (150 r / min) Shake for 10 min to reach adsorption equilibrium, centrifuge, collect the supernatant, and carry out chromatographic analysis. The maximum adsorption capacity of the boron affinity material for catechol was 513.6 μmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com