Alcaftadine intermediate preparation method
A catadine and intermediate technology, which is applied in the field of compound preparation, can solve the problems of unsuitable industrialization and enlargement, large environmental pollution, high equipment corrosiveness, etc., and achieves easy industrial production, easily available raw materials, and short synthesis routes. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
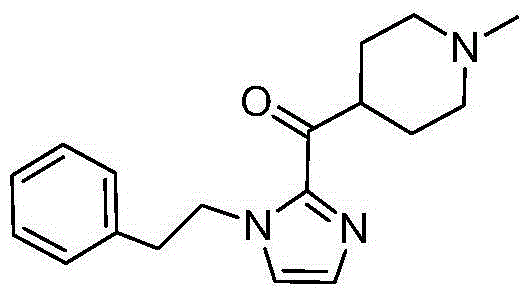
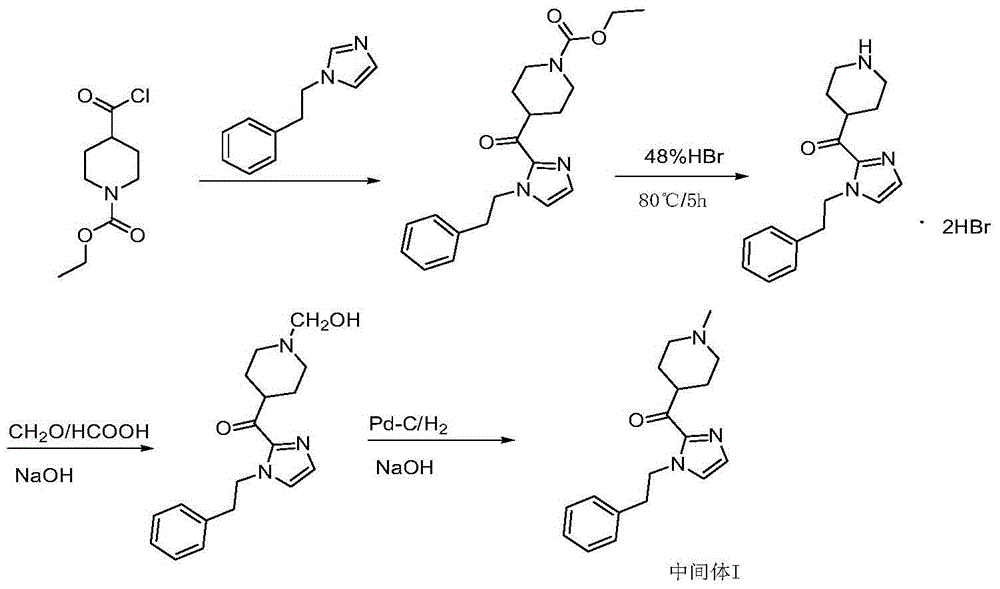
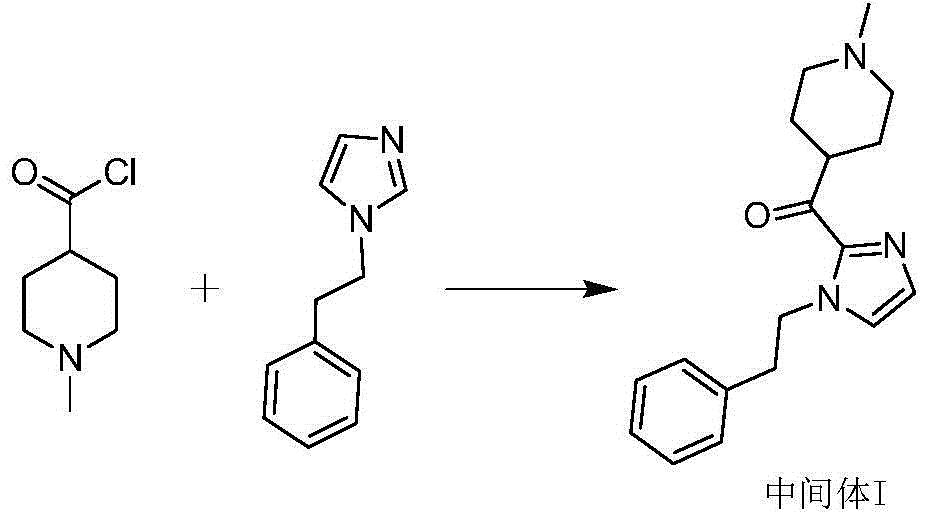
[0032] Preparation of [4-(1-methylpiperidine)][2-(1-phenethyl-1H-imidazole-)] ketone
[0033] Under the protection of nitrogen, dissolve 17.2g (0.1mol) of benethylimidazole in 170mL of tetrahydrofuran, stir and cool to below -40℃, add dropwise 2.5mol / L n-butyllithium n-hexane solution 50mL (0.125mol), and then drop Add 15.7 g (0.1 mol) of methyl 1-methylpiperidine-4-carboxylate, keep stirring at low temperature, monitor by TLC until the reaction is complete, and add glacial acetic acid. The reaction solution was poured into purified water and stirred evenly, extracted with ethyl acetate, the organic layer was separated, dried with anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated under reduced pressure. The obtained oil was added with isopropanol and stirred to precipitate a solid, which was suction filtered and dried to obtain 25.3 g of an off-white solid with a yield of 85.1%. HPLC purity: 98.7%
[0034] 1H NMR 1H NMR (400MHz, CDCl3, TMS): δ7.12...
Embodiment 2
[0036] Preparation of [4-(1-methylpiperidine)][2-(1-phenethyl-1H-imidazole-)] ketone
[0037] Under the protection of nitrogen, dissolve 17.2g (0.1mol) of benethylimidazole in 170mL of tetrahydrofuran, stir and cool down to below -40℃, add dropwise 2.5mol / L n-butyl lithium n-hexane solution 50mL (0.125mol), drip to completion Then, 17.1 g (0.1 mol) of ethyl 1-methylpiperidine-4-carboxylate was added dropwise, and stirring was maintained at low temperature. TLC monitored until the reaction was complete, and glacial acetic acid was added. The reaction solution was poured into purified water and stirred evenly, extracted with ethyl acetate, the organic layer was separated, dried with anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated under reduced pressure. The obtained oily state was stirred with isopropanol to precipitate a solid, which was suction filtered and dried to obtain 24.9 g of an off-white solid with a yield of 83.7% and an HPLC purity of ...
Embodiment 3
[0039] Preparation of [4-(1-methylpiperidine)][2-(1-phenethyl-1H-imidazole-)] ketone
[0040] Under the protection of nitrogen, dissolve 17.2g (0.1mol) of benethylimidazole in 170mL of tetrahydrofuran, stir evenly, cool to below -40°C, dropwise add 2.5mol / L n-butyl lithium n-hexane solution 50mL (0.125mol), Then 18.5 g (0.1 mol) of isopropyl 1-methylpiperidine-4-carboxylate was added dropwise, and the stirring was continued to be maintained at a low temperature. TLC monitored until the reaction was complete, and glacial acetic acid was added. The reaction solution was poured into purified water and stirred evenly, extracted with ethyl acetate, the organic layer was separated, dried with anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated under reduced pressure. The obtained oily substance was added with isopropanol and stirred to precipitate a solid, which was filtered and dried to obtain 26.8 g of off-white solid, yield 90.1%, HPLC purity: 99.2%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com