Long-acting recombinant human brain natriuretic peptide fusion protein and preparation method and thereof and application
A fusion protein and peptide linker technology, which is applied in the fields of molecular biology and medicine, can solve the problems that affect the wide application of products, affect the drug compliance of patients, and short half-life, so as to improve the purity and activity, ensure safety, and high expression yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
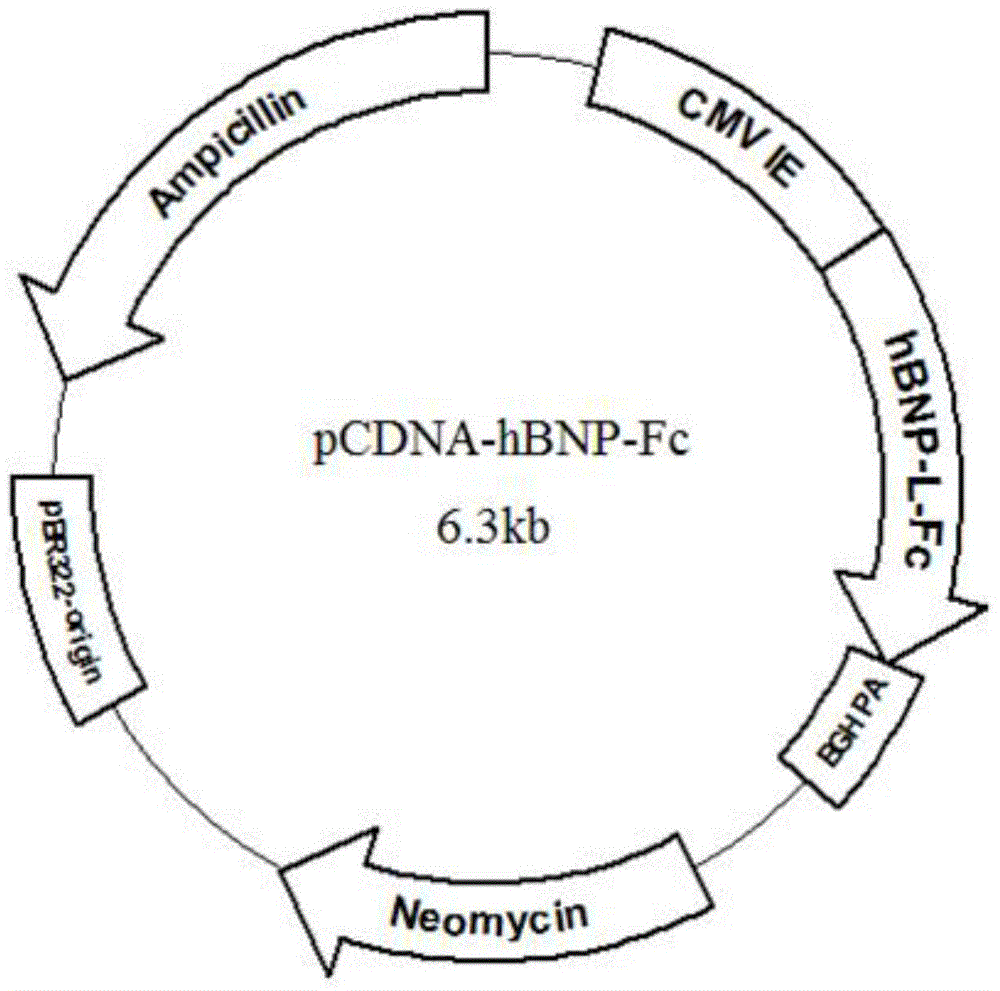
[0056] Example 1. Construction of gene expression plasmids encoding recombinant hBNP-Fc fusion proteins
[0057] (1) Preparation of hBNP gene sequence
[0058] According to the gene sequence of hBNP (Genebank accession number: U34833.1), the gene sequence of the leader peptide and mature protein coding region of hBNP was prepared by artificial synthesis, and the synthetic hBNP gene fragment was inserted into the EcoRV restriction in the transfer vector PUC57. In the restriction site, the phBNP plasmid was obtained, and the correctness of the hBNP gene sequence was verified by DNA sequencing.
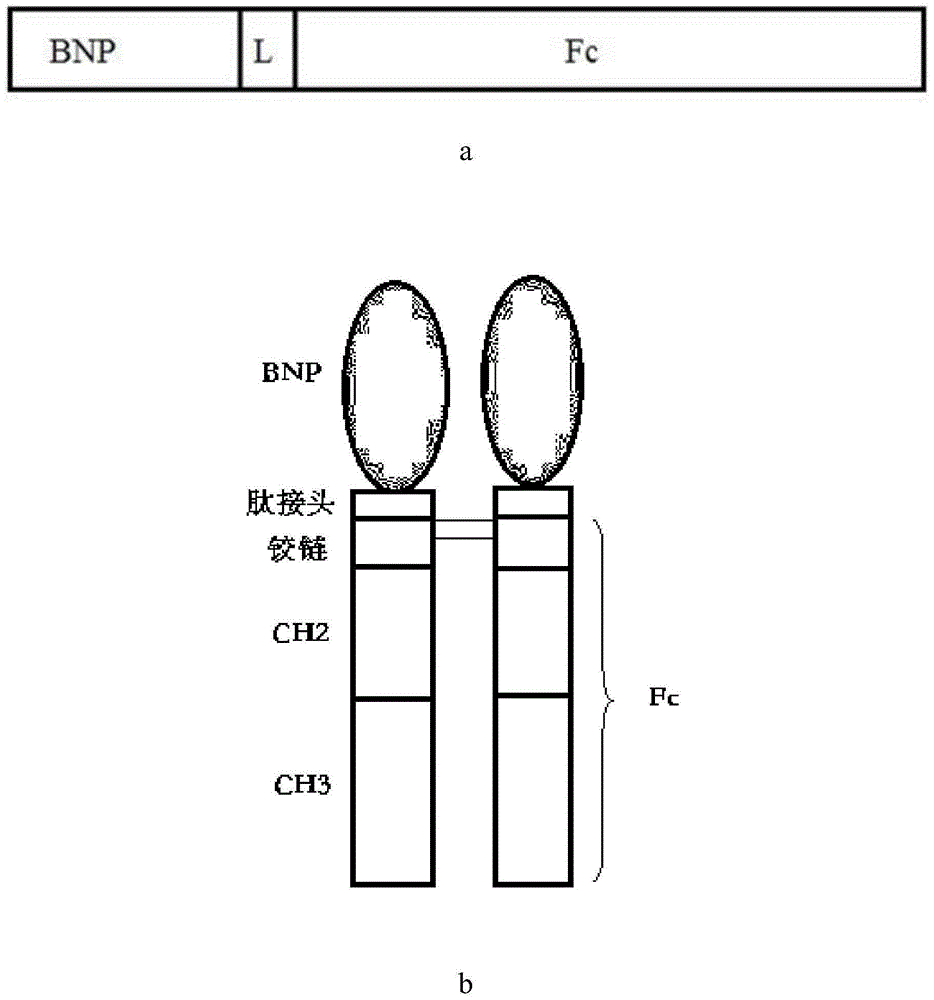
[0059] (2) Preparation of L-vIgG1Fc fusion gene sequence
[0060] The fusion gene L-vIgG4Fc containing the coding peptide linker (Linker, referred to as L) and the human IgG4Fc variant fragment containing the 5' end BamH I and the 3' end EcoR I restriction endonuclease sites were synthesized by artificial synthesis method. The prepared fusion gene fragment was inserted into the BamH I ...
Embodiment 2
[0063] Example 2. Expression of recombinant hBNP-Fc fusion protein in mammalian cells
[0064] The expression plasmid pCDNA-hBNP-Fc constructed in Example 1 and the plasmid pSV2-dhfr (ATCC) containing the DHFR transcription unit were co-transfected into DHFR enzyme-deficient CHO cells (CHO-DHFR - ). Specific steps are as follows:
[0065] Transfection was carried out by electroporation transfection, using a Gene Pulser Electroporator (Bio-Rad Laboratories, Hercules, CA) with a capacity of 900 μFd, the electric field was set at 240 V, and 5 × 10 cells in a cuvette were used. 7 10 μg pCDNA-hBNP-Fc plasmid DNA linearized with Pvu I and 10 μg pSV2-dhfr were added to each cell for co-transfection. After 24 hours of transfection, the culture medium was changed to a growth medium containing 100 μg / mL G418 and 20 nM MTX, and the clones were cloned in a 96-well cell culture plate by limiting dilution to obtain positive clones that had passed the initial resistance screening. The tra...
Embodiment 3
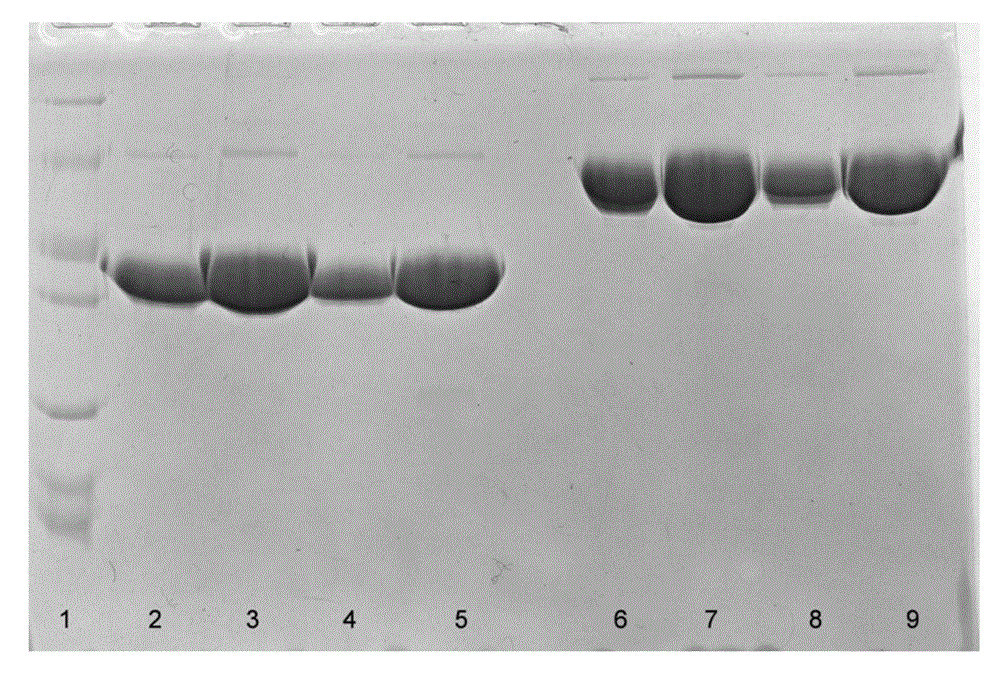
[0067] Example 3. Separation and purification of recombinant hBNP-Fc fusion protein
[0068] (1) Expanded production of CHO cells highly expressing recombinant hBNP-Fc fusion protein
[0069] After the CHO cell line with stable and high expression in Example 2 was fully adapted to serum-free suspension culture, the cells were transferred to a 5L bioreactor for scale-up culture. Select EX-CELL TM 302 serum-free medium (Sigma, 24326C-100L) was used as the basal medium.
[0070] Add ultra-filtered plant peptone (Kerry, HyPep 7504) at a final concentration of 6 g / L or nutrient supplement (Hyclone, Cell Boost 6) at a final concentration of 8 g / L in the basal medium. After 12 days of culture, the expression level of recombinant hBNP-Fc fusion protein accumulated to 1.85g / L.
[0071] (2) Purification of recombinant hBNP-Fc fusion protein;
[0072] a) clarification: adopt the DOHC and B1HC depth filters of Millipore Company to filter the cell culture fluid obtained by the above-m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com