Method for expressing antibacterial peptide apidaecin by using bombyx mori cell and for preparing antibacterial peptide apidaecin
An antibacterial peptide and cell technology, applied in the field of genetic engineering, can solve the problems of high cost, unable to meet the requirements of practical application, low expression amount, etc., and achieve the effects of stable gene expression product, improving expression safety, and increasing expression yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Construction of plasmid and recombinant virus
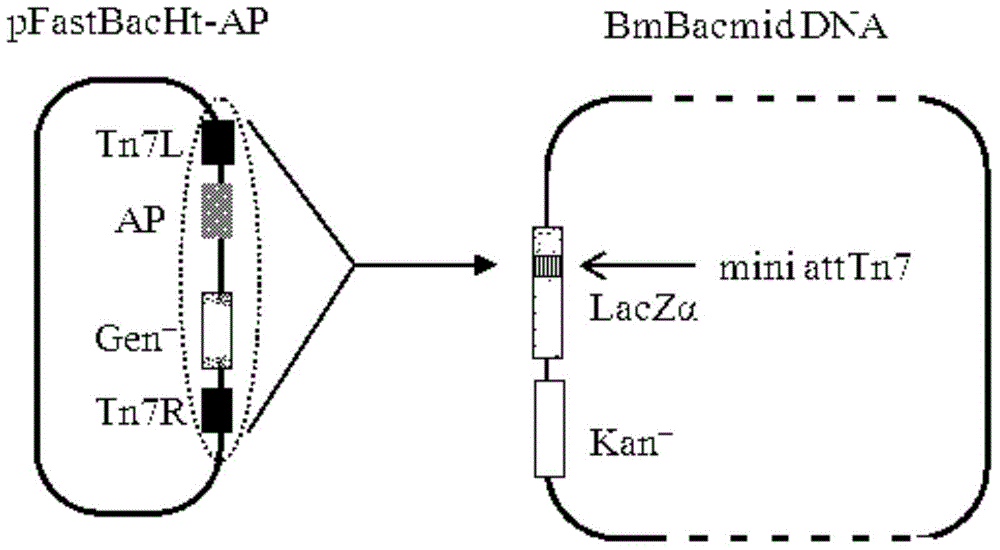
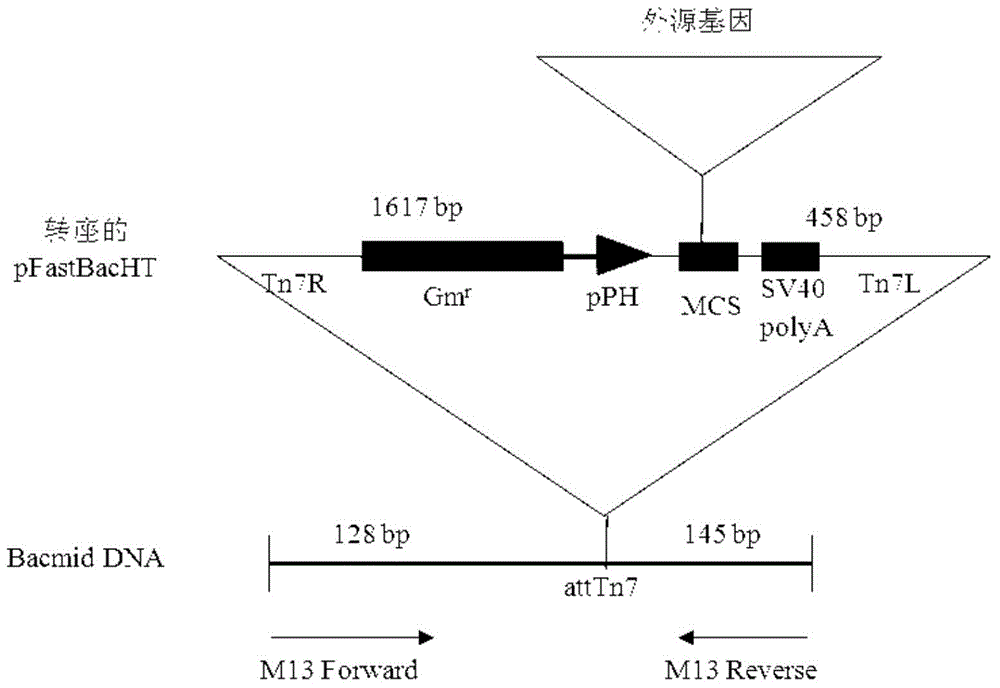
[0037] The AP2 gene fragment was chemically synthesized, the nucleotide sequence of which was described in SEQ ID NO.1, and an enterokinase cleavage site sequence (Asp-Asp-Asp-Asp-Lys) was introduced upstream of the target fragment. The synthesized DNA fragment was directly cloned into the plasmid pFastBacHta to construct the recombinant plasmid pFast-His-AP2, which expressed His-fused AP2.
[0038] Transform the recombinant plasmid into BmDH10Bac competent cells. The specific steps are: add 10 μl of recombinant plasmid ligation mixture to 200 μl competent cells, mix well, place on ice for 30 minutes, place in a 42°C water bath for 45 seconds, and quickly place on ice 2min, then add 800μl S.O.C medium, shake on a shaker at 37°C for 4h, take part of the diluted bacterial solution and spread it on a medium containing kanamycin (50μg / ml), gentamicin (7μg / ml), tetracycline (10μg / ml), X-gal and IPTG on the LB cu...
Embodiment 2
[0049] Embodiment 2: Obtaining of recombinant virus and expression of recombinant protein
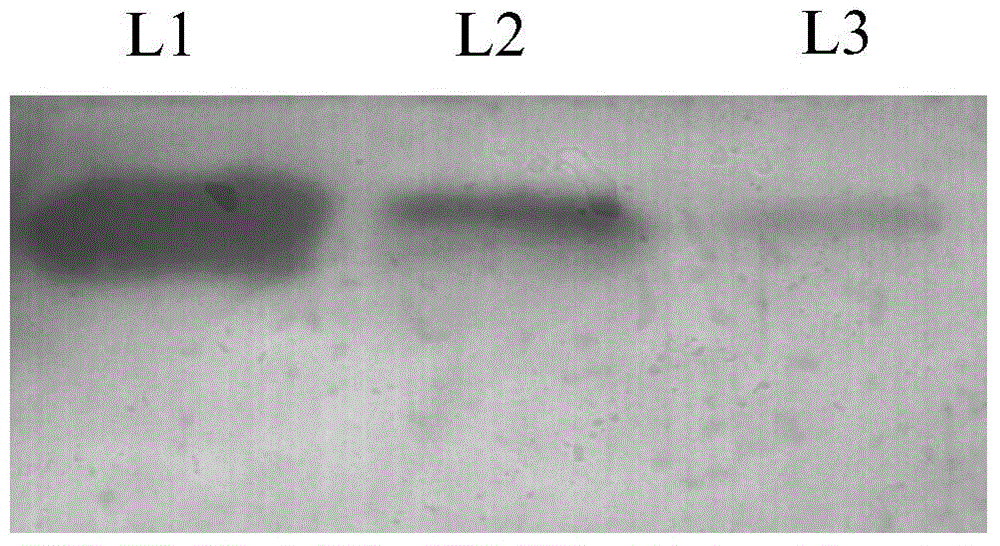
[0050] Take 5 μg of BmBacmid DNA prepared in Example 1 and 5 μl of transfection reagent and fully dissolve them in 100 μl of serum-free TC-100 medium, then mix the two solutions together, place them at room temperature for 30 minutes, and add them to a 35-mm culture dish BmN cells in (cell density 8 × 10 5 / cm 2 , wash twice with serum-free cell culture medium in advance), add 1ml of serum-free TC-100 medium, culture at 27°C for 6h, replace the cells with TC-100 medium containing 10% serum, and continue to culture at 27°C for 96h . After the infection of the cells was seen under a microscope, the supernatant of the infection was collected by centrifugation to obtain the recombinant virus.
[0051] The virus supernatant obtained from the transfection continued to infect the cultured cells for 60 hours, and the infected cells and the culture supernatant were collected separately. The c...
Embodiment 3
[0052] Example 3: Recombinant protein purification and digestion
[0053] Since the N-terminus of the recombinant protein has a 6×His tag tail, it can be separated and purified with a Ni-NTA affinity column under non-denaturing natural conditions. In order to prevent protein degradation, 0.5mM PMSF is kept in the solution of each step of purification. Proceed as follows:
[0054] ① Dilute the supernatant of infected cells sonicated with an equal amount of 2×binding buffer (containing 5mM imidazole) and put it on ice for later use.
[0055] ②Add 2ml Ni-NTA affinity resin to 10ml purification column, wash with 10ml sterilized deionized water, and then equilibrate with 10ml binding buffer (containing 5mM imidazole).
[0056] ③Add the protein sample to be purified, mix by inverting, place flat in an ice-water bath and shake for 30 minutes.
[0057] ④Let the column stand vertically for 10 minutes to allow the resin to settle under the action of gravity, and the supernatant flows ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com