Norbornene imide heat-resistant polymer porous material and preparation method thereof
A technology of bornene imide and porous materials, applied in the field of materials, can solve the problems of high price, poor stability of polyamide carboxylic acid, and limited types of dibasic acid anhydrides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
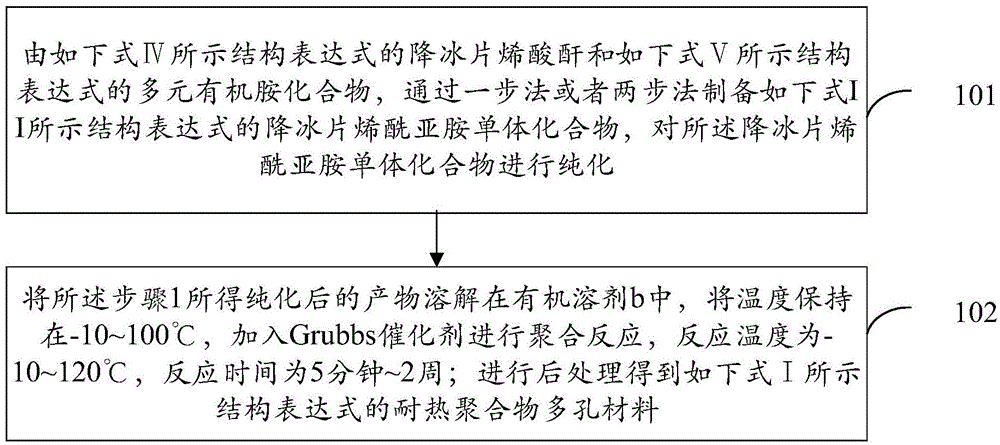
[0038] The invention provides a kind of heat-resistant polymer porous material of norbornene imide and a preparation method thereof. The method comprises two steps: 1. The norbornene imide monomer is prepared by reacting the norbornene anhydride with polyamine compounds; Polymerization reaction, the obtained polymer is washed with a solvent and then dried to obtain a heat-resistant polymer porous material. The present invention utilizes the tension-containing five-membered ring in norbornene and its analogs to easily carry out ring-opening metathesis polymerization reaction under the action of Grubbs catalyst, and the rigidity and non-planar configuration of the pentapentafused ring structure in the reaction product, And the double bond has the characteristics of inhibiting rotation, and introduces a rigid structure, thereby generating non-close packing to ensure the existence of permanent holes. At the same time, the polymerization reaction conditions are relatively mild, an...
Embodiment 1
[0054] Example 1: Hydrazine hydrate linked norbornene imide monomer II(a)
[0055]
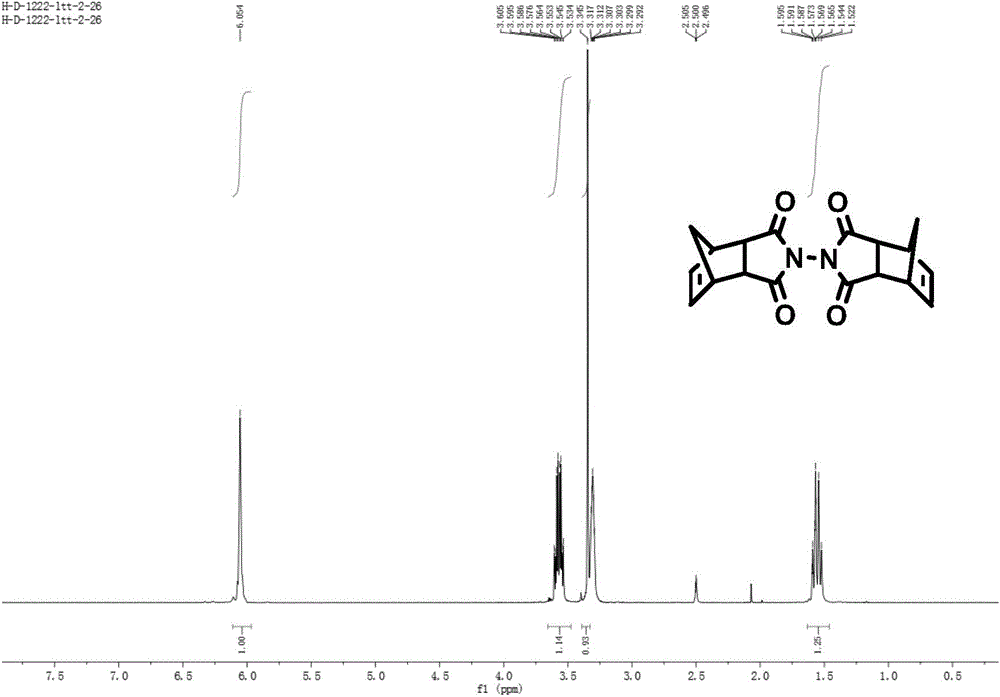
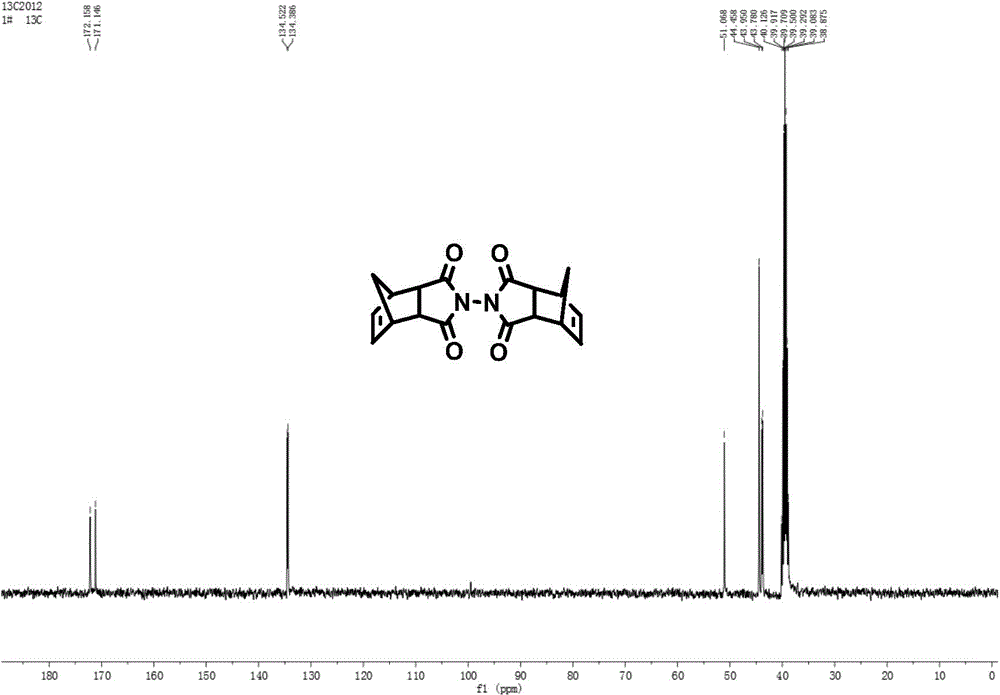
[0056] Add hydrazine hydrate IV (a) (400mg, 4mmol, 1eq, 50%) dropwise to the toluene suspension of NA acid anhydride V(a) (656.64mg, 4mmol, 1eq), stir at room temperature for 20min, TLC shows that the reaction is complete, stop reaction. The solvent was removed under reduced pressure, and the resulting solid product was recrystallized from ethanol to obtain white needle-like crystals, namely NA hydrazide III(a) (581 mg, yield 82%). Rf: 0.26 [V(EtOAc) / V(Petroleum Ether)=2 / 0.5 (2 drops of acetic acid)]; 1 H-NMR (400MHz, CDCl 3 )δ (ppm): 6.09 (s, 2H), 4.12 (s, 2H), 3.38 (s, 2H), 3.24 (s, 2H), 1.75 (d, 1H, J=8.8Hz), 1.54 (d, 1H, J=8.8Hz). The NA hydrazide III (a) (448.3mg, 2.52mmol, 1eq) and NA acid anhydride V (a) (413.7mg, 2.52mmol, 1eq) were added to the acetic acid solvent, heated to reflux for 10h, stopped the reaction, and removed under reduced pressure Acetic acid, the obtained soli...
Embodiment 2
[0062] Example 2: p-Phenylenediamine linked norbornene imide monomer II(b)
[0063]
[0064] NA anhydride V(a) (164.16mg, 1mmol, 1eq) was dissolved in DMF (15ml), p-phenylenediamine (54mg, 0.5mmol, 0.5eq) and triethylamine (101mg, 1mmol, 1eq) were added The reaction system was reacted at 80°C for 2h. After the reaction, the mixture was poured into water, filtered to obtain a solid powder, and recrystallized from acetonitrile to obtain a white solid (184 mg, yield 92%).
[0065] Figure 5 , Figure 6 with Figure 7 Respectively be the proton nuclear magnetic resonance spectrum of II (a) in the embodiment 2, the carbon nuclear magnetic resonance spectrum and the infrared spectrogram.
[0066] H NMR spectrum 1 H NMR (400 MHz, DMSO-d6) δ (ppm): 7.19 (s, 4H), 6.22 (s, 4H), 3.50 (s, 4H), 3.34 (s, 4H), 1.5 (s, 4H).
[0067] C NMR spectrum 13 C-NMR: (100MHz, DMSO-d6) δ (ppm): 176.6, 134.5, 131.9, 127.5, 51.7, 45.4, 44.8.
[0068] Infrared spectrum (KBr, cm -1 ): 2992(w), 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com