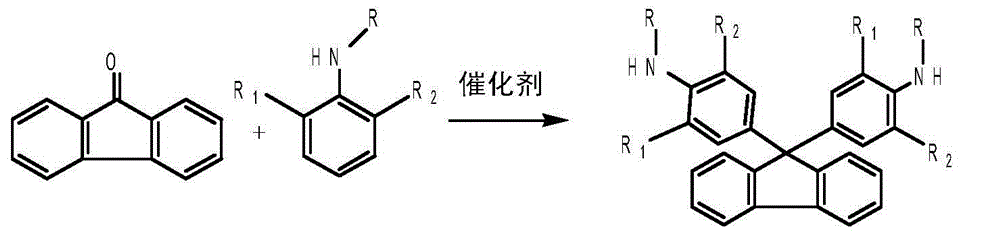
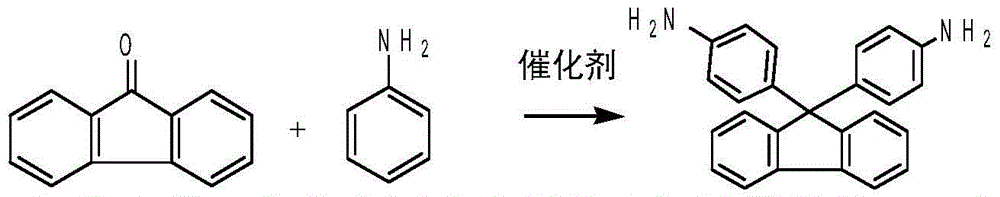
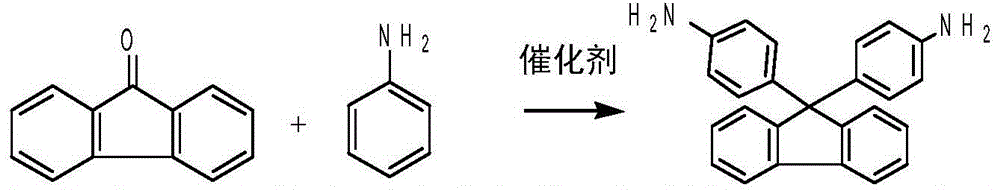
Method for clean preparation of alkyl substituted diamine fluorene compound
A technology for bisamine fluorenes and compounds is applied in the field of clean preparation of alkyl-substituted bisamine fluorenes, which can solve the problems of high product cost and three wastes, and achieve the effects of low cost, cost reduction, and solution to the increase of production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0026] Example 1-1: Preparation of bisamine fluorene from 9-fluorenone
[0027]
[0028] Operation steps: Add 18 grams of 9-fluorenone (0.1mol), 54g (0.58mol) of aniline, 90 grams of toluene, and 1.8 grams of solid superacid SO 4 2- / ZrO(OH) 2 , start stirring, reflux dehydration reaction at 107°C-110°C, track through gas chromatography analysis, when the content of raw material 9-fluorenone is less than 0.1%, the reaction ends; cool down to about 30°C, filter, dry, and prepare white solid diamine fluorene 31.3g, yield 89.9%, purity 99.5%, the solvent used after the reaction is retained, and the next batch continues to be recycled.
[0029] Physical constants and spectral data of bisamine fluorene: melting point: 236-237°C;
[0030] Target compound IR (KBr) (Impact 400 produced by Nicolet, USA): 3533, 3030, 2978, 1610, 1503, 1455, 1283, 812, 740: Target compound NMR (using CDCl 3 CHCl 3 =7.264ppm is the internal standard) (Mercury300 (UX) produced by Varian, USA): δH, ...
Embodiment 1-2
[0031] Example 1-2: Preparation of bisamine fluorene from 9-fluorenone (solvent application)
[0032]
[0033] Operation steps: add 18 grams of 9-fluorenone (0.1mol), 54 g of aniline in a 100 milliliter three-necked flask equipped with a reflux condenser and mechanical stirring, add 10 g of new toluene to 80 grams of toluene reclaimed in the above examples, 1.8 g solid superacid SO 4 2- / ZrO(OH) 2 , start stirring, reflux dehydration reaction at 110°C-113°C, track through gas chromatography analysis, when the content of raw material 9-fluorenone is less than 0.1%, the reaction ends; cool down, filter, and dry to prepare 31g of white solid diamine fluorene, the yield 89.1%, purity 99.5%, the solvent used after the reaction is retained, and the next batch continues to be recycled.
Embodiment 2
[0034] Example 2: Preparation of bis-o-methylamine fluorene from 9-fluorenone
[0035]
[0036] Operation steps: Add 18 grams of 9-fluorenone (0.1mol), 85.6g (0.8mol) o-toluene, 180 grams of toluene, 0.36 grams of solid supernatant in a 100 milliliter three-necked flask equipped with a reflux condenser and mechanical stirring Strong acid SO 4 2- / ZrO(OH) 2 , start stirring, reflux dehydration reaction at 110°C, track through gas chromatography analysis, when the content of raw material 9-fluorenone is less than 0.1%, the reaction ends; cool down, filter, and dry to prepare 33.1g of white solid diamine fluorene, with a yield of 88% , the purity is 99%, the solvent used after the reaction is retained, and the next batch continues to be recycled.
[0037] Bis-o-methylamine fluorene physical constants and spectral data: melting point: 230-232°C;
[0038] Target compound IR (KBr) (Impact 400 produced by Nicolet, USA): 3400, 3033, 2958, 1599, 1503, 1456, 1278, 806, 743: Target ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com