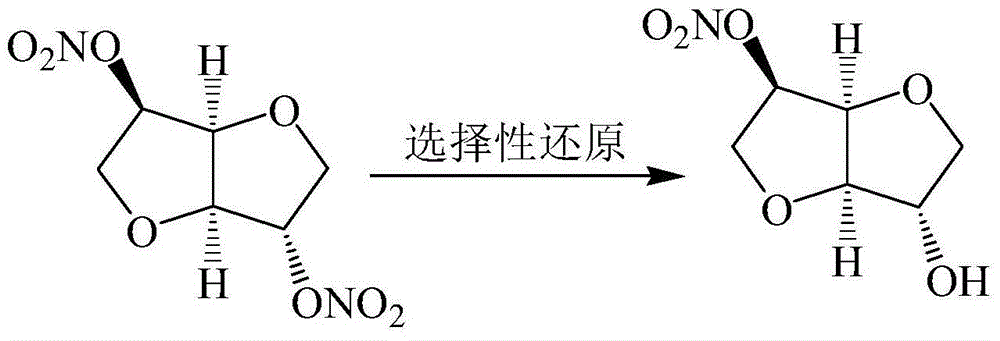
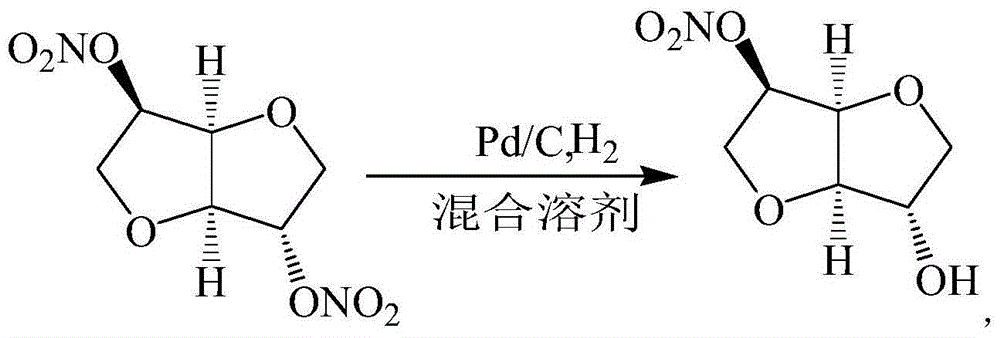
Preparation method for isosorbide 5-mononitrate
A technology of isosorbide dinitrate and nitro, applied in the field of medicine, can solve the problems of high industrialization cost, poor catalyst reduction selectivity, high catalyst cost, etc., and achieve the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Dissolve 12.14g (0.12mol) of triethylamine in a mixture of 1350mL of ethanol and 150mL of purified water, add it to the high-pressure hydrogenation autoclave, stir for 10min, then add 100.00g (0.42mol) of isosorbide dinitrate and 1.50g of 10% Add Pd / C into the high-pressure hydrogenation kettle, lower the temperature and stir, wait until the temperature drops to about 0°C, maintain the hydrogen pressure at 0.3 MPa, and continue the reaction for 12 hours. Quantitative detection and analysis of the product (except a very small amount of unreacted isosorbide dinitrate) obtained: 1.5% of isosorbide 2-mononitrate, 82.4% of isosorbide 5-mononitrate, 16.1% of isosorbide.
[0039] Finally, the concentrated solution was extracted with ethyl acetate, the extract was washed 2-3 times with 0.2mol / L hydrochloric acid, then washed with water until neutral, dried with anhydrous magnesium sulfate, and the ethyl acetate was distilled off under reduced pressure to obtain 5- 66.00 g of is...
Embodiment 2
[0041] 4.25g (0.04mol) triethylamine was dissolved in the mixed solution of 900mL ethanol and 100mL purified water, it was added in the high pressure hydrogenation kettle, stirred for 10min, then 100.00g (0.42mol) isosorbide dinitrate and 1.50g10% Add Pd / C into the high-pressure hydrogenation kettle, lower the temperature and stir, wait until the temperature drops to about 0°C, maintain the hydrogen pressure at 0.3 MPa, and continue the reaction for 12 hours. Extract with ethyl ester, wash the extract with 0.2mol / L hydrochloric acid for 2-3 times, then wash with water until neutral, dry with anhydrous magnesium sulfate, evaporate ethyl acetate under reduced pressure to obtain isosorbide 5-mononitrate crystals 44.00g, the purity of 5-isosorbide mononitrate detected by liquid phase is 97.40%, and the yield is 54.37%.
Embodiment 3
[0043] Dissolve 12.14g (0.12mol) of triethylamine in a mixture of 1350mL of ethanol and 150mL of purified water, add it into a high-pressure hydrogenation autoclave, stir for 10min, then add 100.00g (0.42mol) of isosorbide dinitrate and 2.00g of 10% Add Pd / C into the high-pressure hydrogenation kettle, lower the temperature and stir, wait until the temperature drops to about 5°C, maintain the hydrogen pressure at 1.0 MPa, and continue the reaction for 9 hours. Extract with ethyl ester, wash the extract with 0.2mol / L hydrochloric acid for 2-3 times, then wash with water until neutral, dry with anhydrous magnesium sulfate, evaporate ethyl acetate under reduced pressure to obtain isosorbide 5-mononitrate crystals 56.70g, the purity of 5-isosorbide mononitrate detected by liquid phase is 98.40%, and the yield is 70.06%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com