Programmed multi-targeting dendrimer assembly drug delivery system and its preparation method and application
A technology of dendritic macromolecules and delivery systems, which is applied in the field of biomedical materials and can solve the problems that the nano-delivery system has not been properly solved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
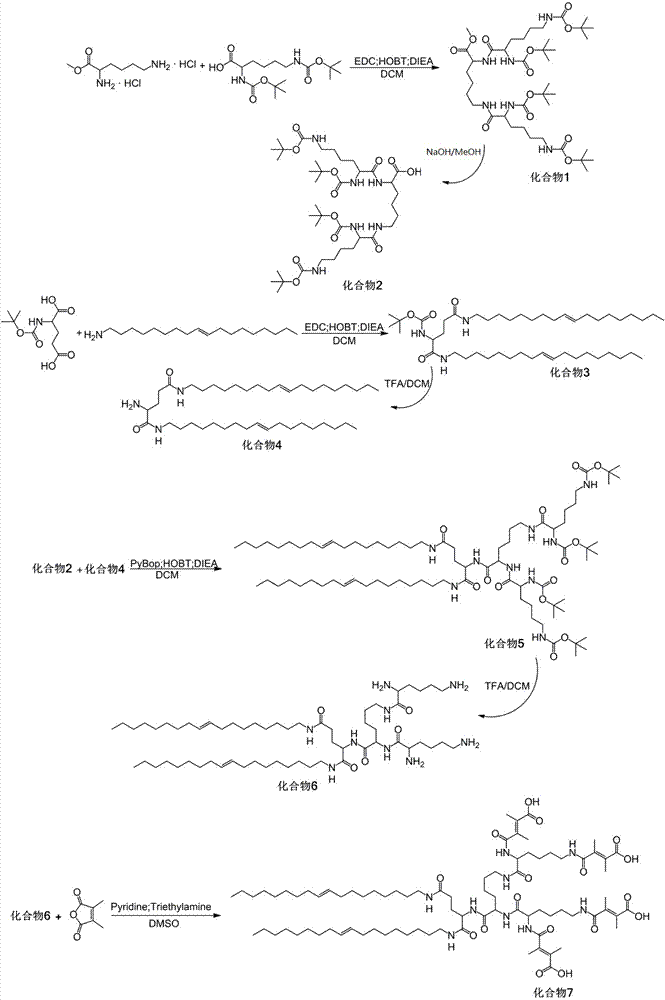
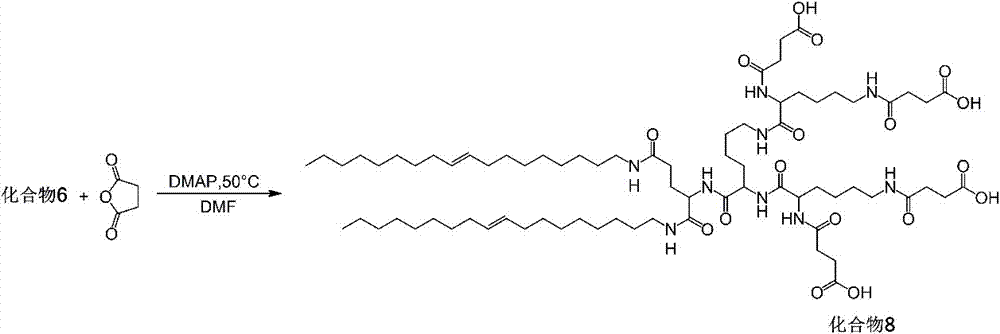
[0073] Example 1: Preparation of programmed multi-targeted amphiphilic dendrimer self-assembly motif 1 (synthetic route as shown in figure 1 )
[0074] Second-generation lysine dendrimers (HO-Lys(G2)-Boc 4 )Synthesis
[0075] Weigh 4.0 g of H-Lys-OMe·2HCl, 14.8 g of Boc-Lys(Boc)-OH, 5.1 g of HOBT and 13.2 g of EDC·HCl in a 100 mL round-bottomed flask with a branch tube, vacuumize, fill with nitrogen, add 30 mL of redistilled DCM was stirred to dissolve, and 28.3 mL of DIEA was added to react for 0.5 h under ice-cooling, and then reacted at room temperature for 48 h. The solvent was removed by rotary evaporation with a water pump, dissolved in chloroform, and washed successively with 1 M HCl, saturated NaHCO 3 After washing with NaCl, anhydrous MgSO 4 After drying overnight, filter, spin off the solvent, and separate by 200-300 mesh silica gel column chromatography (eluent DCM / EA = 1:1) to obtain white powder MeO-Lys(G2)-Boc 4 (compound 1).
[0076] Demethylation protecti...
Embodiment 2
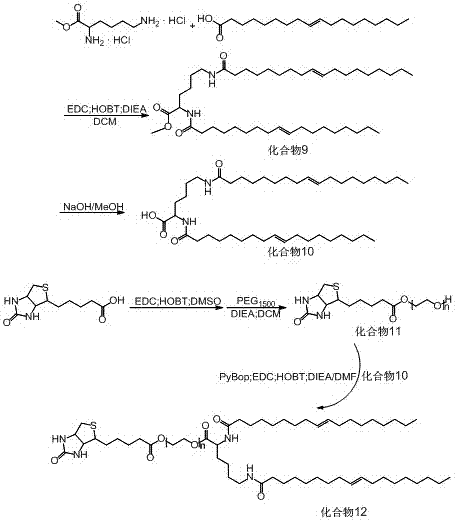
[0087] Example 2: Preparation of programmed multi-targeted amphiphilic dendrimer self-assembly motif II (synthetic route as shown in image 3 )
[0088] Functionalized hydrophilic end (PEG 1500 -Biotin) synthesis
[0089] Accurately weigh 1.0 g of biotin, 1.6 g of EDC·HCl and 0.6 g of HOBT in a 250 mL round bottom flask with a branch tube, vacuumize, fill with nitrogen protection, and add 20 mL of DMSO to dissolve. After biotin was activated for 2 h, add 12.2 g PEG 1500 and 3.4 mL of DIEA in DCM for 24 h at room temperature. After the oil pump was rotated to remove the solvent, the residue was dissolved in chloroform and washed with saturated NaHCO 3 After washing with NaCl, anhydrous MgSO 4 After drying overnight, filter, spin off the solvent, and separate by 200-300 mesh silica gel column chromatography to obtain white solid PEG 1500 -Biotin (compound 11).
[0090] Synthesis of Hydrophobic End of Oleic Acid-Lys-OH
[0091] Accurately weigh 5.0 g of H-Lys-OMe·2HCl, 16...
Embodiment 3
[0095] Example 3: Determination of Amphiphilic Dendrimer Self-Assembly Motif-Critical Aggregation Concentration
[0096] First prepare a concentration of 6.02 × 10 -7 mol / L aqueous solution of pyrene, and then dilute the amphiphilic dendrimer with end group functionalization prepared in Example 1 with the above prepared pyrene water to a concentration of 1.0 mg / mL to 1.0 × 10 -7 mg / mL solutions with different concentrations. The emission wavelength was fixed at 395 nm, the excitation wavelength in the range of 300 nm - 380 nm was measured with a fluorescence spectrophotometer, and the fluorescence values at 338 nm and 334 nm were recorded. with I 338 / I 334 The ratio of is the ordinate, and the concentration is the abscissa, and the result is as follows Figure 5 shown. The measured critical aggregation concentration is 4.85 μg / mL, and the smaller critical aggregation concentration value indicates that the assembly has better potential stability and prevents disassembl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com