Bougainvillea glabra triterpenoid saponin, hpyerglycemic drugs with triterpenoid saponin as active component and preparation method and application thereof
A kind of technology of leaf leaf flower and triterpenoid saponins, which is applied in the preparation of hypoglycemic drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
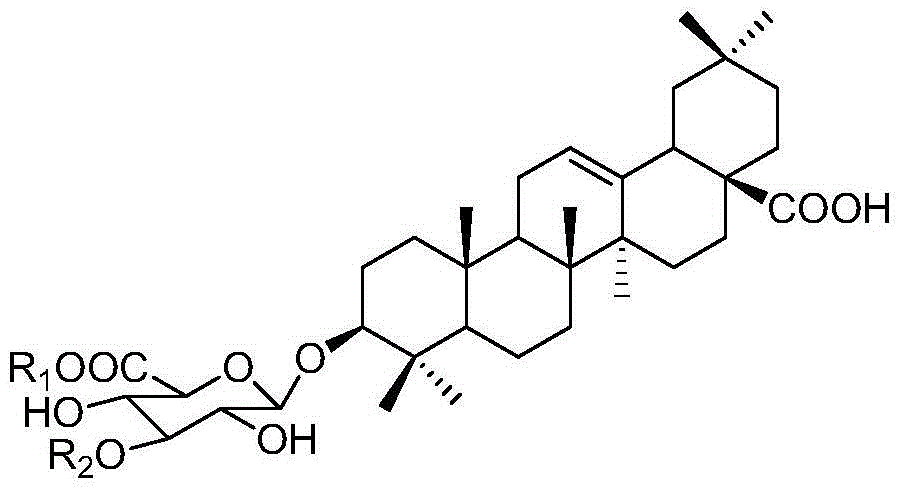
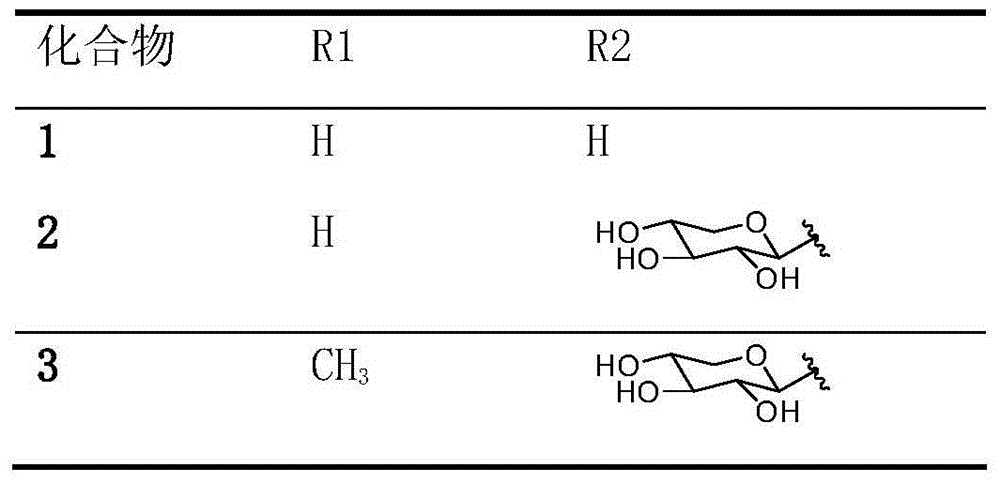
[0037] The triterpenoid saponins compounds oleanolic acid-3-O-β-D-glucopyranoside (1), oleanolic acid-3-O-β-D-xylopyranose (1 →3)-β-D-glucopyranose (2), oleanolic acid-3-O-β-D-xylopyranose (1→3)-β-D-glucopyranose Preparation and structure identification of methyl glucoside (3):
[0038] Take the leaves and leaves (50 kg), dry and pulverize them, and extract them by cold soaking with 90% methanol at room temperature for 3 times, the time is 7, 3, and 3 days each time, and the extract is concentrated under reduced pressure to obtain methanol extract ; After the methanol extract is suspended in water, it is fully extracted with ethyl acetate and n-butanol successively, and the equal volume is extracted three times respectively, and the solvent is recovered to obtain the ethyl acetate part, n-butanol part and water part; Column chromatography, 100:0, 20:1, 10:1, 8:1, 6:1, 2:1, 0:100 chloroform / methanol gradient elution, thin layer chromatography, combined with triterpene saponins...
Embodiment 2
[0051] The triterpene saponin compound of the present invention, glabrata saponins, oleanolic acid-3-O-β-D-glucopyranoside (1), oleanolic acid-3-O-β-D-xylopyranose ( 1→3)-β-D-glucopyranose (2), oleanolic acid-3-O-β-D-xylopyranose (1→3)-β-D-glucopyranose Acid methyl glycoside (3) has a therapeutic effect on streptozotocin (STZ)-induced hyperglycemia in mice with type 2 diabetes. The experimental principles, methods and results are as follows:
[0052] Experimental principle: Streptozotocin (STZ) selectively destroys the islet β cells of mice, causing them to develop diabetes. The mice were fed with high-calorie feed for a certain period of time and fasted for 12 hours, and STZ was injected intraperitoneally at 100 mg / kg body weight. , can prepare type Ⅱ diabetes animal model, and the model prepared according to this method has the characteristics of overweight, impaired glucose tolerance, elevated blood lipid, elevated serum insulin and decreased insulin receptor binding capac...
Embodiment 3
[0061] Compounds obtained in Example 1 Oleanolic acid-3-O-β-D-glucopyranose (1), oleanolic acid-3-O-β-D-xylopyranose (1→3) -β-D-glucopyranose (2), oleanolic acid-3-O-β-D-xylopyranose (1→3)-β-D-glucopyranose methyl (3), add 4% sulfuric acid ethanol solution, pH = 4, filter and dry to prepare sulfate compound 1-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com