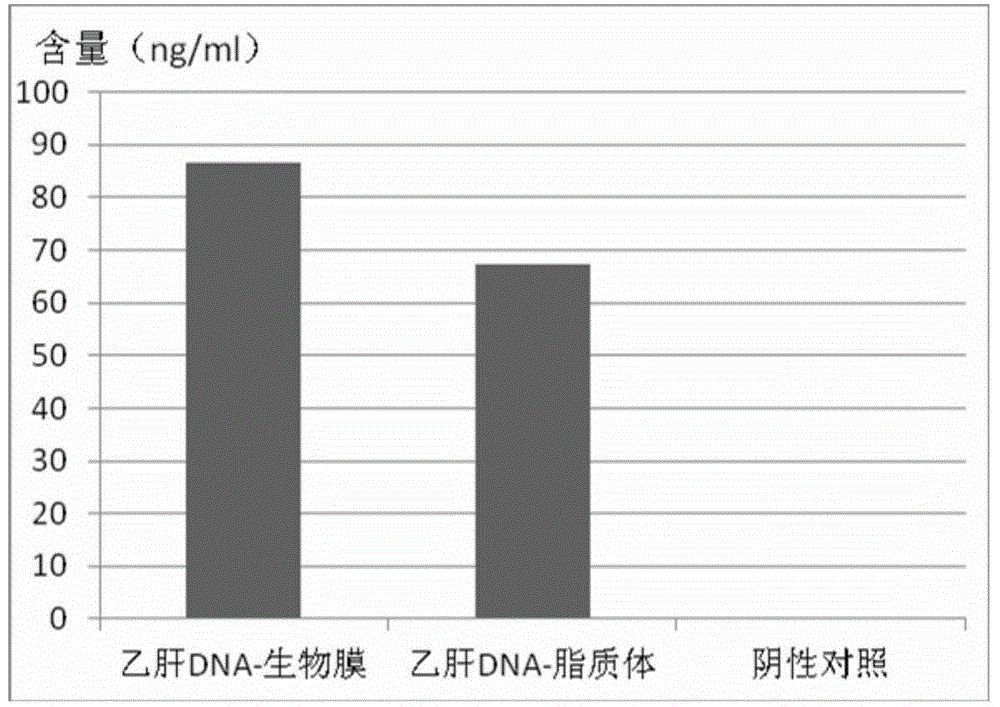
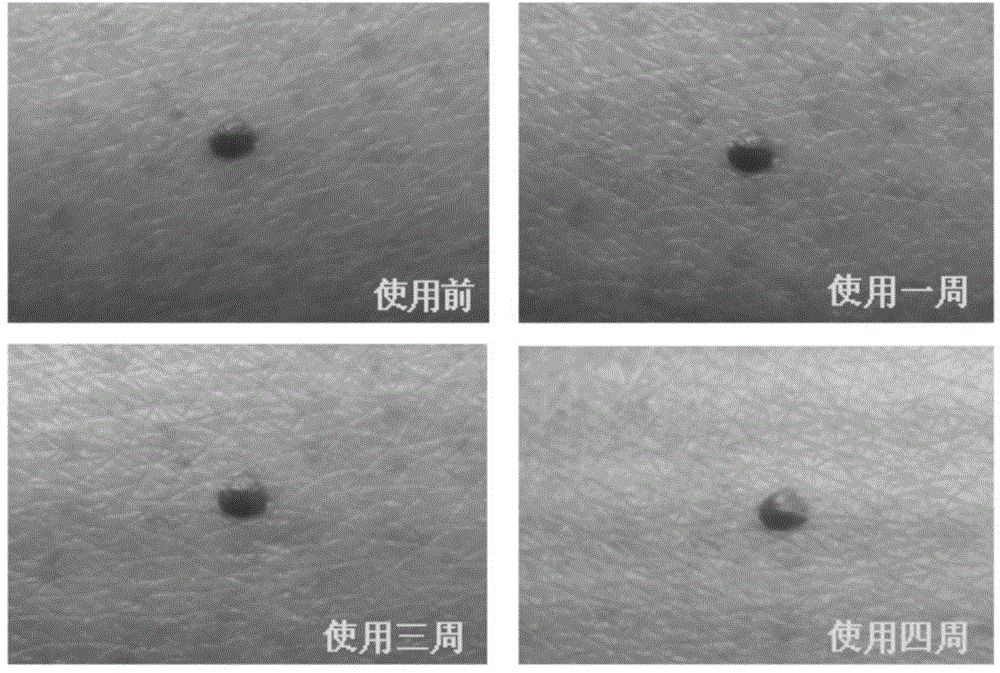
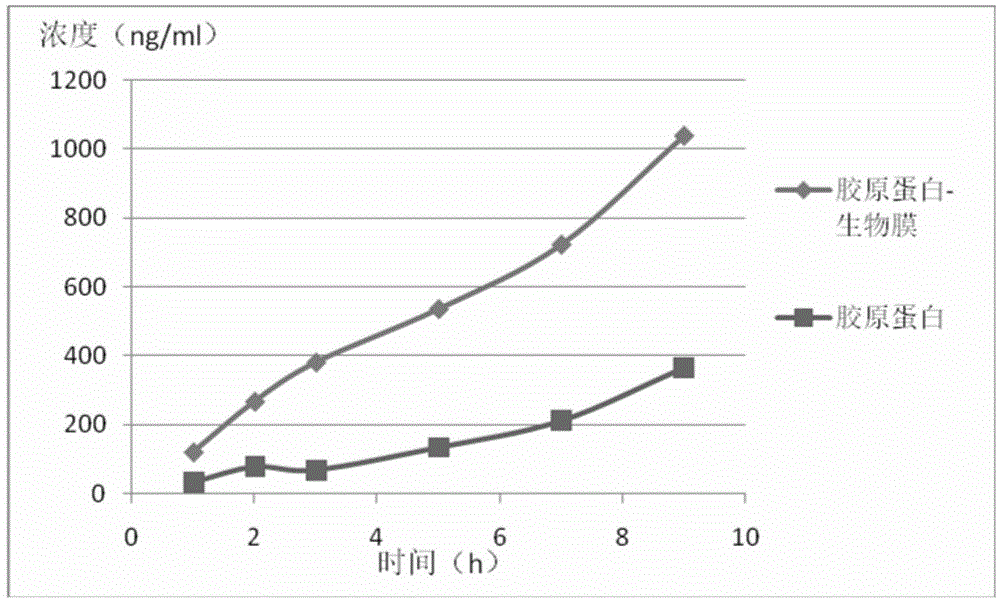
Application of naturally-sourced and/or self-assembled biological membrane, closed structure or cell compartment with biological membrane property as medical high polymer material
A self-assembly technology, a technology of polymer materials, applied to other methods of inserting foreign genetic materials, recombinant DNA technology, medical preparations of non-active ingredients, etc., to achieve high safety, rich technical support, and high utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Extraction and purification of biofilm
[0043] The desired biofilm was extracted and purified by density gradient centrifugation. The specific process is as follows:
[0044] (1) New Zealand white rabbits that had been fasted for 18-24 hours were sacrificed to take the liver, and after removing the large blood vessels, the liver was cut into 2mm 3 The left and right small pieces were washed with normal saline until the tissue pieces turned white;
[0045] (2) Preparation of homogenate: 1mmol / L CaCl 2 , 50mmol / L HEPES (pH 7.4), 1mmol / L PMSF, 2μg / mL Aprotinin, 2μg / mL Antipain;
[0046] (3) Add liver tissue and homogenate to 8 times the volume of homogenate according to the mass:volume ratio, and homogenize with an electric homogenizer at 15,000 rpm in an ice-bath environment until the tissue is liquefied;
[0047] (4) Filter the homogenate through four layers of gauze, centrifuge the filtrate at 1,000×g, 10min, 4°C, and collect the precipitate;
[0048] ...
Embodiment 2
[0050] Example 2 Extraction and purification of biofilm
[0051] The desired biofilm was obtained by extracting and purifying by two-phase partition method. The specific process is as follows:
[0052] (1) Select plump and consistent normal corn seeds, soak them in 1% NaClO for 10 minutes, rinse them, and germinate them at 25°C in constant temperature and darkness for 72 hours;
[0053] (2) Take corn epicotyl, add 2 times the volume of extraction buffer (5mmol / L EDTA, 25mmol / L Tris, 0.25mmol / L sucrose, 1mmol / L MgSO 4 , 0.2% (W / V) BSA, 0.5% (W / V) PVP-10, 10% (W / V) glycerol, 15mmol / L β-mercaptoethanol, 1mmol / L PMSF, 1mmol / L DTT), ice bath grinding to liquefaction;
[0054] (3) Filter the grinding solution through four layers of gauze, centrifuge the filtrate at 12,000×g, 15min, 4°C, and take the supernatant;
[0055] (4) Centrifuge the supernatant at 80,000×g for 30 minutes at 4°C to collect the precipitate;
[0056] (5) Precipitate with suspension (25mmol / L Tris, 0.25mmo...
Embodiment 3
[0058] Example 3 Extraction and purification of biofilm
[0059] The desired biofilm was obtained by extracting and purifying by differential centrifugation. The specific process is as follows:
[0060] (1) Antarctic ice algae ( Chlamydomonas subcaudata ) is isolated and purified from Antarctic sea ice;
[0061] (2) Inoculate Antarctic ice algae into the medium at a ratio of 1:100 (21.2g NaCl, 3.6g NaSO per 10L medium 4 , 0.6g KCl, 0.3g NaHCO 3 , 0.1g KBr, 0.1g H 3 BO 3 , 0.1g NaF, 9.6g MgCl 2 ·6H 2 O, 1.0 g CaCl 2 , 0.1g SrCl 2 ·6H 2 O), cultured in a controlled light incubator. Cultivate for 14 days at -4°C, with a light intensity of 1300~1900lx, a light cycle of 12 light / 12 dark, and shaking 4~5 times a day;
[0062] (3) Centrifuge the ice algae culture solution at 4,000rpm, 20min, and 4°C to collect the ice algae sediment, and rinse it twice quickly with pre-cooled distilled water to remove extracellular viscous, surface salt and impurities in the culture so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com