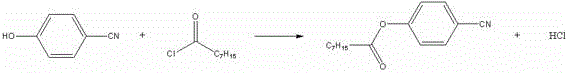
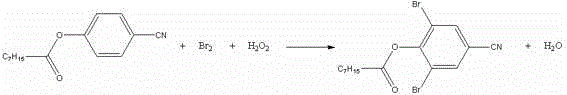
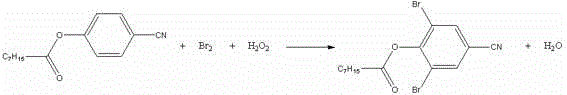
Preparation process for bromoxynil octanoate
A technology for the preparation of bromoxynil octanoyl, which is applied in the field of bromoxynil octanoyl preparation, can solve the problems of low degree of production automation, the need to recycle bromine mother liquor, and environmental pollution accidents, so as to reduce the incidence of pollution accidents , reduce production costs, and avoid the effect of dry powder feeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] In a 250ml four-necked bottle, add 23.8g (0.2mol) of p-hydroxybenzonitrile and 33.2g (0.204mol) of octanoyl chloride, raise the temperature to 105±5°C, keep it warm for 3 hours, take a sample, and after the reaction is qualified, evaporate the excess octanoyl chloride. acid chloride. After steaming octanoyl chloride, cool down to 50±5°C, add 120ml of dichloroethane and 0.5g of tetra-n-butylammonium bromide, control the temperature at 50±5°C, add dropwise 27.2g (0.4mol) of 50% hydrogen peroxide and 32g (0.2mol) of bromine, dropwise for about 4 hours, after the dropwise addition is completed, keep warm for 30 minutes, take a sample for testing, after the reaction is qualified, add a small amount of alkali to destroy the hydrogen peroxide, use starch potassium iodide test paper to pass the test, heat up to remove the solvent, When the temperature rises to 95°C, stop the distillation, add 100ml of process water, keep warm at 90°C for 30 minutes, pour it into a separatory fu...
Embodiment 2
[0020] In a 250ml four-necked bottle, add 23.8g (0.2mol) p-hydroxybenzonitrile and 33.2g (0.204mol) octanoyl chloride, raise the temperature to 105±5°C, keep it warm for 3 hours, take a sample, after the reaction is qualified, evaporate the excess octanoyl chloride. After steaming octanoyl chloride, cool down to 50±5°C, add 120ml cyclohexane and 0.5g tetra-n-butylammonium bromide, control the temperature at 50±5°C, add dropwise 27.2g (0.4mol) 50% hydrogen peroxide and 32g (0.2mol) bromine, about 4 hours of dripping time, after the dropwise addition, keep warm for 30 minutes, take a sample and detect, after the reaction is qualified, add a small amount of alkali, destroy hydrogen peroxide, pass the test with starch potassium iodide test paper, heat up and remove the solvent, wait The temperature rises to 95°C, stop the distillation, add 100ml of process water, keep warm at 90°C for 30 minutes, pour it into a separatory funnel, wash the lower organic phase with water, separate t...
Embodiment 3
[0022] In a 250ml four-necked bottle, add 23.8g (0.2mol) p-hydroxybenzonitrile and 33.2g (0.204mol) octanoyl chloride, raise the temperature to 105±5°C, keep it warm for 3 hours, take a sample, after the reaction is qualified, evaporate the excess octanoyl chloride. After steaming octanoyl chloride, cool down to 50±5°C, add 120ml of methanol and 0.5g of tetra-n-butylammonium bromide, control the temperature at 50±5°C, add dropwise 27.2g (0.4mol) of 50% hydrogen peroxide and 32g (0.2 mol) bromine, the dropping time is about 4 hours, after the dropping is completed, keep warm for 30 minutes, take a sample test, after the reaction is qualified, add a small amount of alkali, destroy the hydrogen peroxide, use the starch potassium iodide test paper to test qualified, heat up to remove the solvent, wait for the temperature to rise To 95°C, stop the distillation, add 100ml of process water, keep warm at 90°C for 30 minutes, pour it into a separatory funnel, wash the lower organic pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com