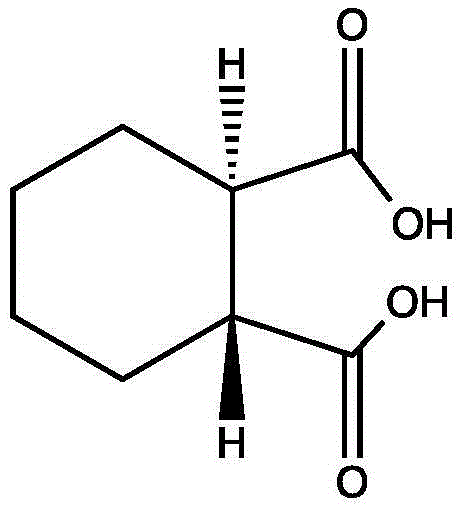
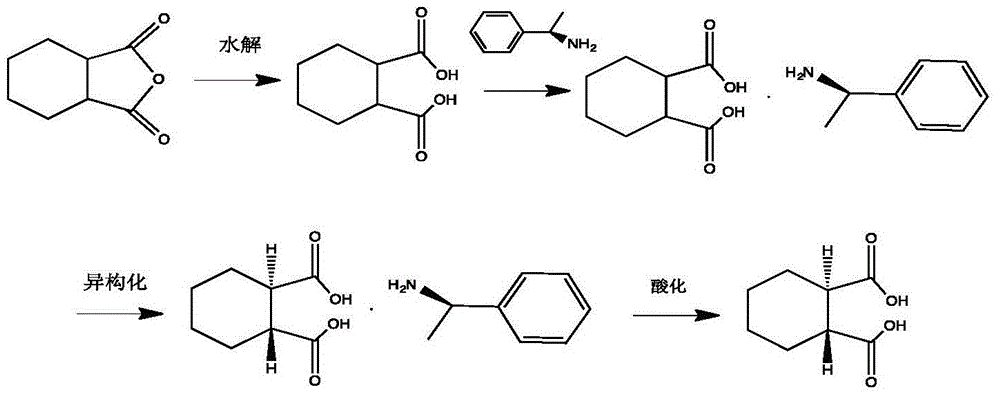
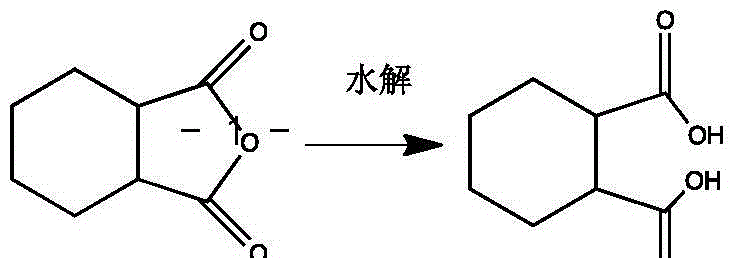
Synthesis method of (1R, 2R)-trans-cyclohexane dicarboxylic acid
A technology of cyclohexanedicarboxylic acid and synthesis method, which is applied in the field of compound synthesis, can solve the problems of long steps, low yield, and many waste liquids, and achieve high recovery rate, high crystallization efficiency, and high reaction conversion rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Take 15g of cis-hexahydrophthalic anhydride and 150ml of 70% sulfuric acid solution in a reaction flask, heat to reflux at 120°C for 26 hours, neutralize with 10% sodium hydroxide, add activated carbon at the same time, stir for 1 hour, filter, and the filtrate is neutralized with hydrochloric acid To pH = 2, stirred for 1 h, filtered to obtain a white granular product of a mixture of cis-1,2-cyclohexanedicarboxylic acid and (1R,2R)-trans-cyclohexanedicarboxylic acid (15:85) 10.1g.
[0027] The mixture in the previous step was heated to reflux with 4 times of isopropyl ether for 1 hour, then lowered to room temperature, and solids were slowly precipitated during the cooling process, stirred at room temperature for 2 hours, filtered, and dried to obtain a crude product with higher purity, and then Then heated to reflux with 4 times of isopropyl ether to dissolve, lowered to room temperature, stirred for 2 hours, filtered, and dried to obtain 6.4 g of white particles (1R,...
Embodiment 2
[0029] Take 15g of cis-hexahydrophthalic anhydride and 300ml of 48% hydrobromic acid solution in a reaction flask, heat at 120°C for 18 hours, neutralize with 10% sodium hydroxide, add activated carbon at the same time, stir for 1 hour, filter, and the filtrate is neutralized with hydrochloric acid and to pH=2, stirred for 1h, filtered to obtain a mixture of white granular product cis-1,2-cyclohexanedicarboxylic acid and (1R,2R)-trans-cyclohexanedicarboxylic acid (HPLC, 14 :86) 11.0 g.
[0030] The mixture in the previous step was heated to reflux with 4 times of isopropyl ether for 1 hour, then lowered to room temperature, and solids were slowly precipitated during the cooling process, stirred at room temperature for 2 hours, filtered, and dried to obtain a crude product with higher purity, and then Then heated to reflux with 4 times isopropyl ether to dissolve, lowered to room temperature, stirred for 2 hours, filtered, and dried to obtain 7.2 g of (1R,2R)-trans-cyclohexaned...
Embodiment 3
[0032] Take 15g of cis-hexahydrophthalic anhydride and 300ml of 48% acetic acid solution in a reaction flask, heat at 120°C for 20 hours, neutralize with 10% sodium hydroxide, add activated carbon at the same time, stir for 1 hour, filter, and neutralize the filtrate with hydrochloric acid to pH=2, stirred for 1h, filtered to obtain a mixture of white granular product cis-1,2-cyclohexanedicarboxylic acid and (1R,2R)-trans-cyclohexanedicarboxylic acid (HPLC, 20:80 ) 11.2g.
[0033] The mixture in the previous step was heated to reflux with 4 times of isopropyl ether for 1 hour, then lowered to room temperature, and solids were slowly precipitated during the cooling process, stirred at room temperature for 2 hours, filtered, and dried to obtain a crude product with higher purity, and then Then heated to reflux with 4 times isopropyl ether to dissolve, lowered to room temperature, stirred for 2 hours, filtered, and dried to obtain 6.1 g of (1R,2R)-trans-cyclohexanedicarboxylic ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com