Method for preparing Moxifloxacin impurity C
A moxifloxacin and impurity technology, applied in the field of medicinal chemistry, can solve problems such as no efficient synthesis method of moxifloxacin impurity C, and achieve the effect of short synthesis route, simple operation and high purity of impurity products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
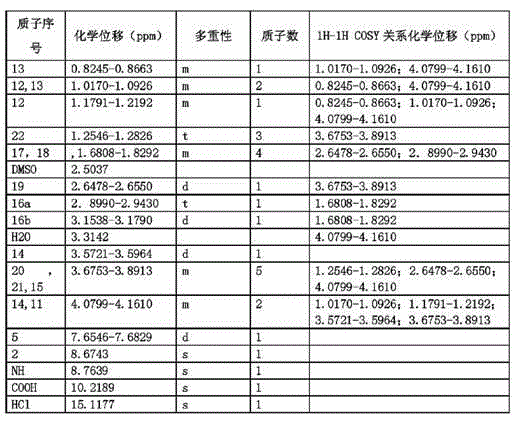
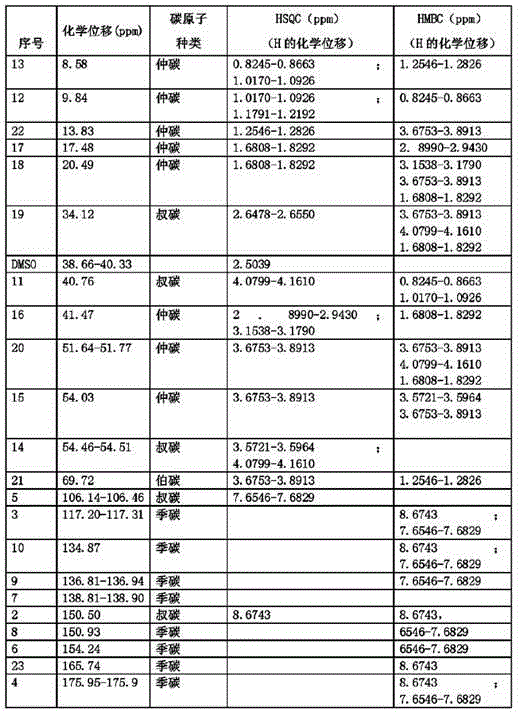
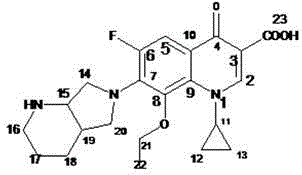
[0033] Dissolve moxifloxacin impurity E (20.0g, 0.052mol) in 220mL of a mixed solvent of 1,4-dioxane / water with a volume ratio of 1:1, add sodium hydroxide (1.0g, 0.026mol), and wait Lower the internal temperature to 0°C, slowly add di-tert-butyl dicarbonate (Boc) dropwise 2 O (12.4g, 0.057mol) in 1,4-dioxane solution, after dropping, keep the internal temperature at 0°C for 2 hours, slowly rise to the internal temperature of 30°C, continue to stir for 12 hours, after the reaction is completed, cool down to 0 ℃, add 0.5mol / L citric acid aqueous solution dropwise to pH = 3, add dichloromethane, stir and extract, stand and separate layers, take the dichloromethane layer, concentrate to dryness, and obtain 21.4g of the compound of formula III, with a yield of 85% .
[0034] Dissolve the compound of formula III (21.4g, 0.04mol) in 100mL of anhydrous THF, add potassium hydroxide (8.9g, 0.16mol), stir evenly, wait until the internal temperature drops to 0-5°C, slowly add the ethyla...
Embodiment 2
[0040] Dissolve moxifloxacin impurity E (15g, 0.038mol) in 150mL of 1,4-dioxane / water mixed solvent with a volume ratio of 1:1, add sodium hydroxide (2.3g, 0.057mol), and wait for The temperature dropped to 5°C, and slowly added dropwise (Boc) 2 O (10.7g, 0.049mol) in 1,4-dioxane solution, after dripping, keep the internal temperature at 5°C for 2-3 hours, slowly rise to the internal temperature of 35°C, continue stirring for 12-14 hours, after the reaction is completed , cooled to 5°C, added dropwise 0.5mol / L citric acid aqueous solution to pH = 3, added dichloromethane, stirred and extracted, stood to separate layers, took the dichloromethane layer, concentrated to dryness, and obtained 15.6g of the compound of formula III, The yield is 83%.
[0041] Dissolve the compound of formula III (15.6g, 0.032mol) in 95mL of anhydrous THF, add sodium hydride (5.1g, content 60%, 0.128mol), stir evenly, wait until the internal temperature drops to 0-5°C, slowly add Ethylating reagent ...
Embodiment 3
[0047] Dissolve moxifloxacin impurity E (10 g, 0.026 mol) in 95 mL of a mixed solvent of 1,4-dioxane / water with a volume ratio of 1:1, add sodium hydroxide (1.4 g, 0.036 mol), and wait for Lower the temperature to 0~5℃, slowly add (Boc) 2 O (6.7g, 0.031mol) of 1,4-dioxane solution, after dropping, keep the internal temperature at 3°C for 2.5h, slowly rise to the internal temperature of 33°C, continue stirring for 13h, after the reaction is complete, cool down to 0~5°C, add 0.5mol / L citric acid aqueous solution dropwise to pH=3, add dichloromethane, stir and extract, stand and separate layers, take the dichloromethane layer, concentrate to dryness, and obtain 10.2g of the compound of formula III. The rate is 81%.
[0048] Dissolve the compound of formula III (10.2g, 0.021mol) in 100mL of anhydrous THF, add sodium ethoxide (5.7g, 0.084mol), stir evenly, wait until the internal temperature drops to 0-5°C, slowly add the ethylating reagent dropwise Bromoethane (9.1g, 0.084mol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com