Novel compound of anti-infection drug and preparation method thereof
A drug compound and anti-infection technology, applied in the field of medicine, can solve the problems of easy residue, high stability, poor fluidity, etc., and achieve the effects of reducing impurities and moisture content, improving stability and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: a kind of preparation method of anti-infective drug compound, the steps are as follows:
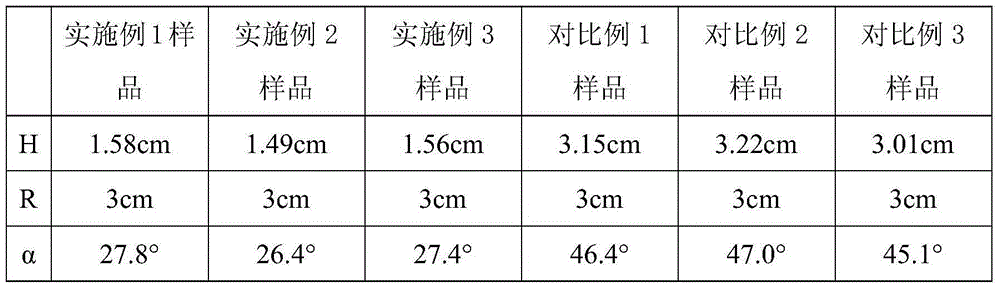
[0032] Dissolve 1.0Kg of cefazedone sodium solid in 4.0L of pure aqueous solution at 15°C; first add a mixed solvent of isopropanol and acetone with a total volume of 8.0L, and the volume ratio of isopropanol to acetone is 1:1, Stir while adding, control the temperature at 15°C, and grow the crystal for 0.5 hours; then add a mixed solvent of isopropanol and acetone with a total volume of 12.0L, the volume ratio of isopropanol and acetone is 1:1, and grow the crystal for 2 hours. Hours later, the temperature was lowered to 5°C at a speed of 5°C / hour, and then the stirring speed was kept at 80 rpm to stir and crystallize and grow crystals for 1 hour; filter, dry at 40°C, and dry under reduced pressure to obtain 0.958Kg of cefazedone sodium crystalline compound, The yield was 95.8%.
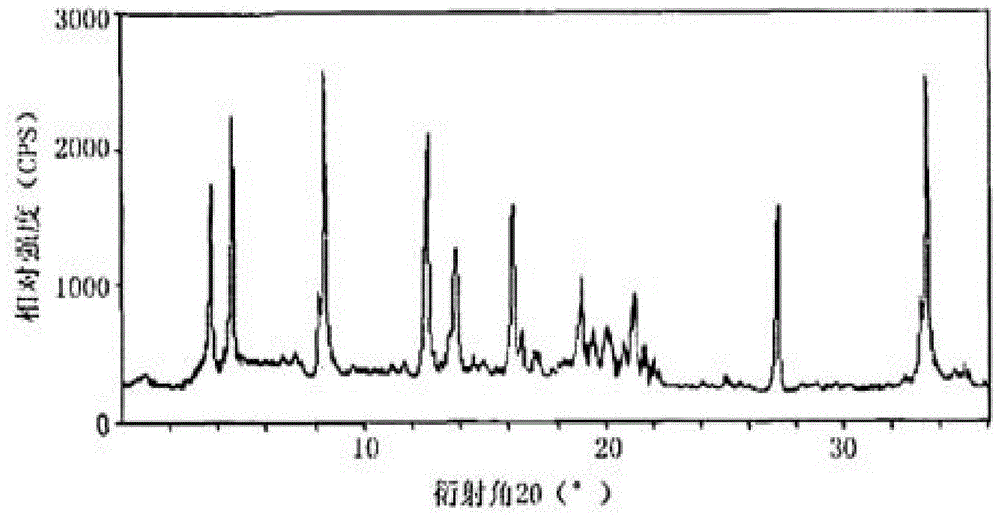
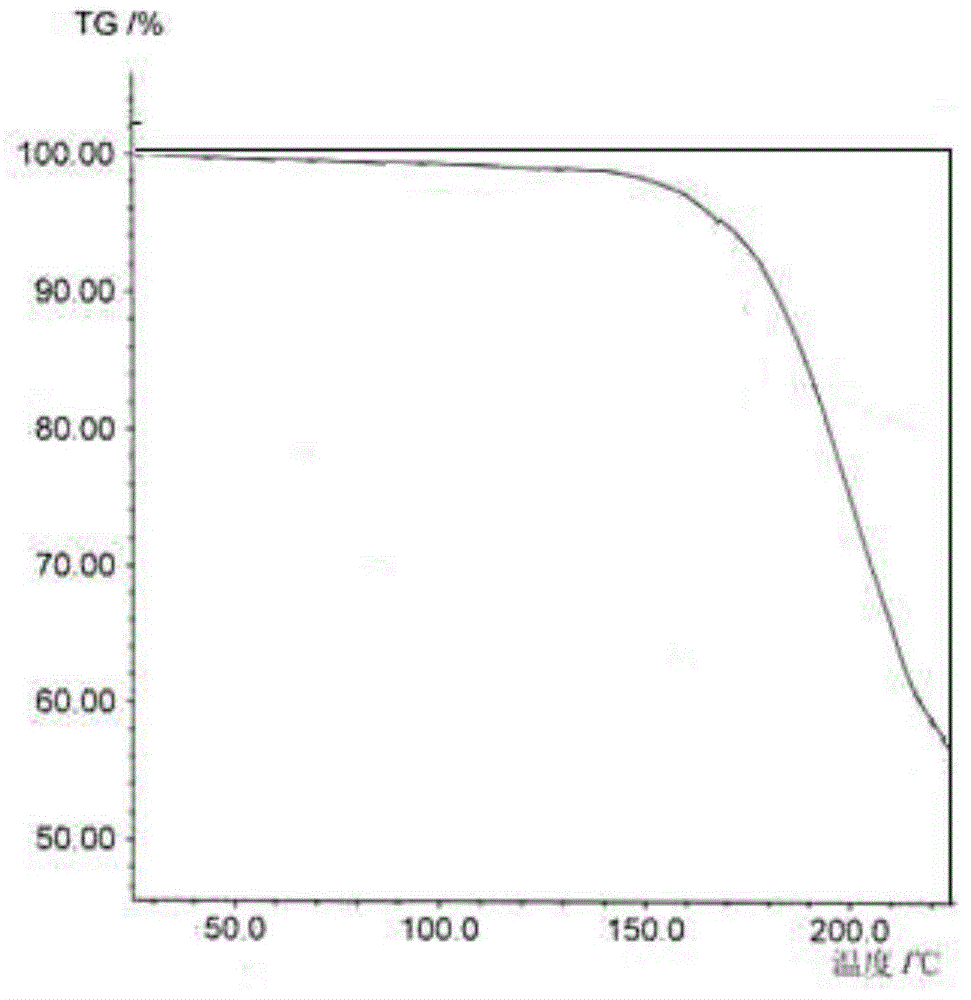
[0033] The prepared X-ray powder diffraction pattern obtained by measuring the cefazed...
Embodiment 2
[0034] Embodiment 2: a kind of preparation method of anti-infective drug compound, the steps are as follows:
[0035] Dissolve 1.0Kg of cefazedone sodium solid in 8.0L of pure aqueous solution at 35°C; first add a mixed solvent of anhydrous isopropanol and anhydrous acetone with a total volume of 10.0L at a rate of 4ml / min, and anhydrous isopropanol The volume ratio of propanol to anhydrous acetone is 1:1, stir while adding, control the temperature at 35°C, grow the crystal for 1 hour; then add anhydrous isopropanol with a total volume of 25.0L at a speed of 4ml / min , a mixed solvent of anhydrous acetone, the volume ratio of anhydrous isopropanol to anhydrous acetone is 1:1, after growing crystals for 5 hours, cool down to 5°C at a speed of 15°C / hour, and then maintain a stirring speed of 120 rpm / The crystallization was stirred for 3 hours, and the crystal was grown for 3 hours; filtered, 50° C., and dried under reduced pressure for 12 hours to obtain 0.925 Kg of cefazedone s...
Embodiment 3
[0037] Embodiment 3: a kind of preparation method of anti-infective drug compound, the steps are as follows:
[0038] Dissolve 1.0Kg of cefazedone sodium solid in 6.0L of pure aqueous solution at 25°C; first add a mixed solvent of anhydrous isopropanol and anhydrous acetone with a total volume of 9.0L at a rate of 3ml / min, and anhydrous isopropanol The volume ratio of propanol to anhydrous acetone is 1:1, stir while adding, control the temperature at 25°C, and grow crystals for 0.7 hours; then add anhydrous isopropanol with a total volume of 19.0L at a speed of 3ml / min , a mixed solvent of anhydrous acetone, the volume ratio of anhydrous isopropanol to anhydrous acetone is 1:1, after growing crystals for 3.5 hours, cool down to 5°C at a speed of 10°C / hour, and then maintain a stirring speed of 100 rpm / The crystallization was stirred for 2 hours, and the crystal was grown for 2 hours; filtered, 45° C., and dried under reduced pressure for 11 hours to obtain 0.936 Kg of cefazedon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com