Ionic liquid-lithium salt gel polymer electrolyte and preparation and application thereof
A technology of gel polymers and ionic liquids, which is applied in the manufacture of hybrid capacitor electrolytes and hybrid/electric double layer capacitors, etc., can solve the problems of wide electrochemical window, good cycle stability, and increased crystallinity, and achieve high ion density. Conductivity, improve flexibility, weaken the effect of damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
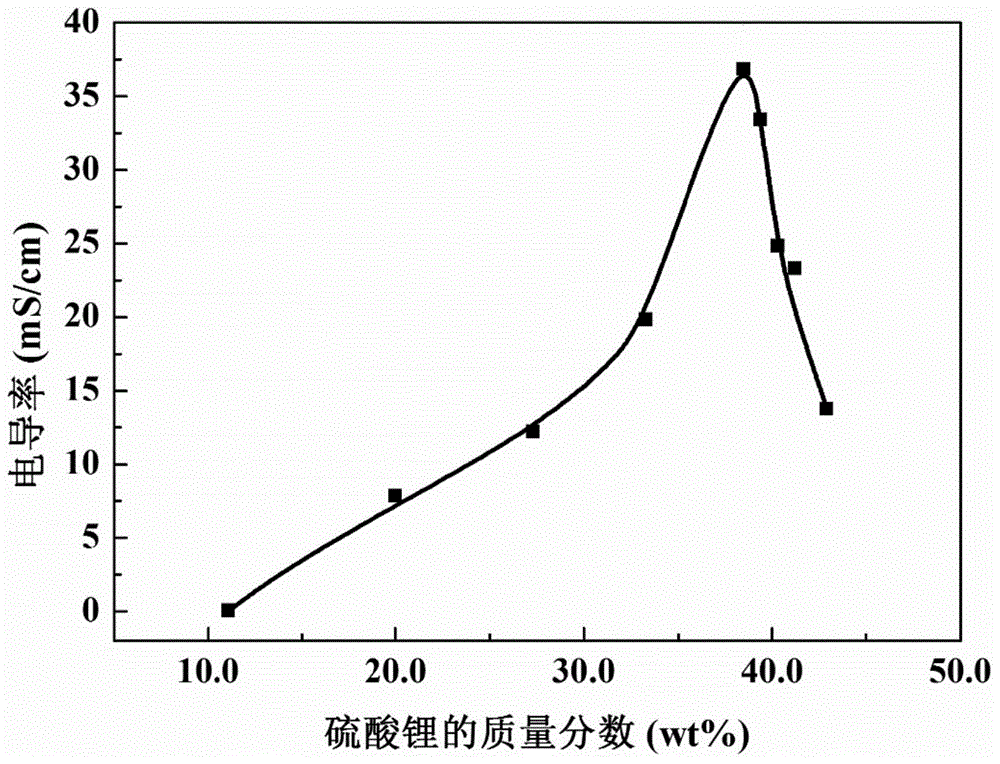
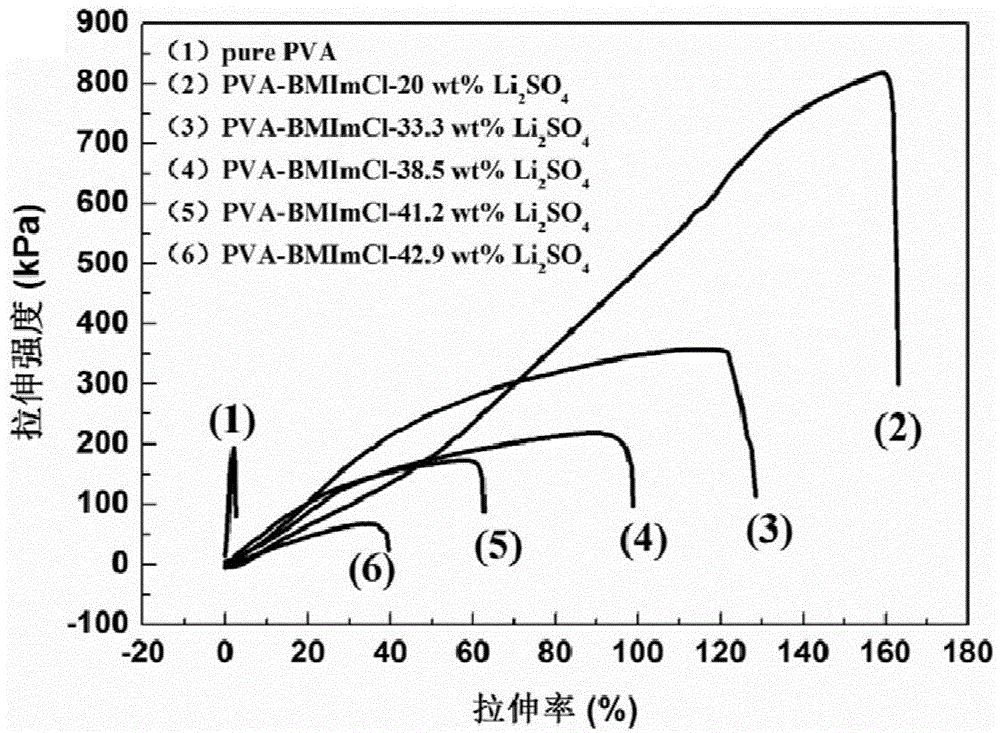
[0043] Embodiment 1, different Li 2 SO 4 ContentPVA-BMImCl-Li 2 SO 4 Preparation of Gel Polymer Electrolyte and Measurement of Its Conductivity
[0044] 1) Prepare a 10wt% PVA aqueous solution, place it in a 90°C oven for 24 hours, and form a uniformly dispersed PVA aqueous solution;
[0045] 2) Take out 10g of the 10wt% PVA aqueous solution prepared in step 1), add 3.0g of BMImCl ionic liquid, stir at 50°C, mix 0.5g, 1.0g, 1.5g, 2.0g, 2.5g, 2.6g, 2.7g, 2.8g, 3.0gLi 2 SO 4 were dissolved in 15g of deionized water to prepare a solution, and then different concentrations of Li 2 SO 4 The solution was added to the corresponding PVA-BMImCl system, and stirred at 50°C for 30min;
[0046] 3) After transferring the mixed solution obtained in step 2) into a petri dish, move it into the cold trap of a lyophilizer to freeze for 60min, and then freeze-dry for 24h to prepare the PVA-BMImCl-Li 2 SO 4 Gel polymer electrolyte.
[0047] The prepared PVA-BMImCl-Li 2 SO 4 Gel polym...
Embodiment 2
[0049] Embodiment 2, the preparation of active carbon electrode.
[0050] 1) The nickel foam was dried by ultrasound for 24 hours, cut into discs with a radius of 7.5 mm using a circular mold, and recorded its initial mass m1.
[0051] 2) According to activated carbon: acetylene black: polyvinylidene fluoride (PVDF) = 8:1:1 (mass ratio), take the sample in an agate mortar, add an appropriate amount of N-methylpyrrolidone (NMP) dropwise and grind it into a paste shape. And spread it evenly on the nickel foam cut out in 1), and put it in a 60°C oven to dry for 24h.
[0052] 3) After taking out the electrode sheet in 2), press it with a pressure of 40MPa, and record its mass m 2 . Calculate the activated carbon mass of the coated electrode material, and the average mass of each electrode sheet is 8 mg.
Embodiment 3
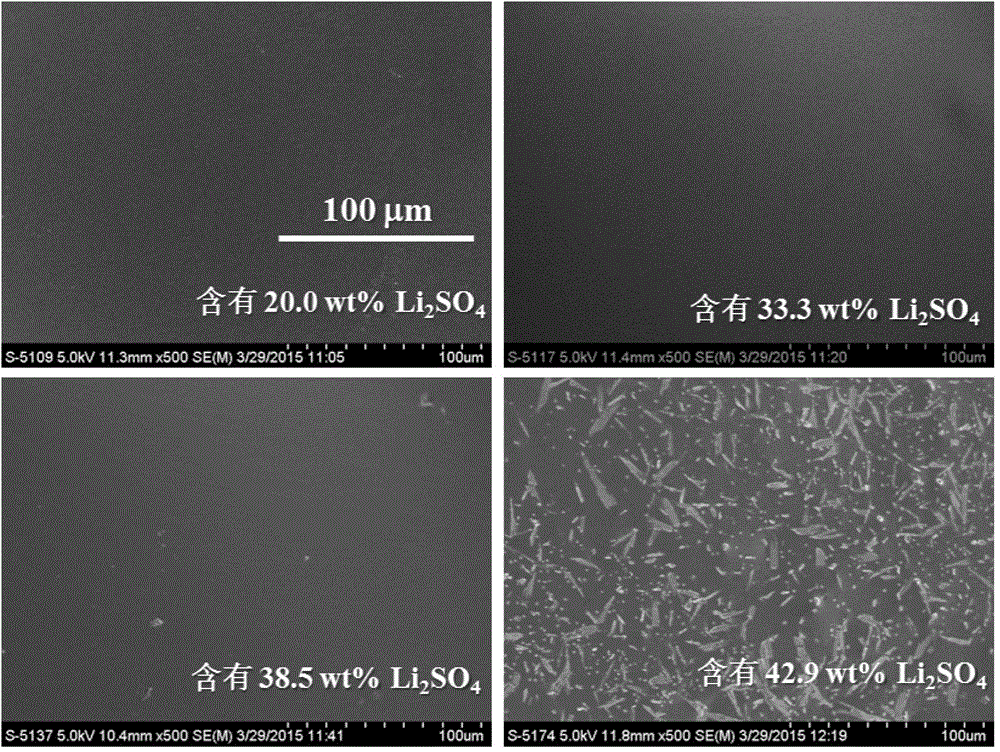
[0053] Embodiment 3, different Li 2 SO 4 ContentPVA-BMImCl-Li 2 SO 4 Preparation and Morphology Characterization of Gel Polymer Electrolyte
[0054] 1) Consistent with the preparation method of Example 1, Li 2 SO 4 PVA-BMImCl-Li with content of 20.0wt%, 33.3wt%, 38.5wt%, 42.9wt% 2 SO 4 Gel polymer electrolyte;
[0055] 2) The prepared PVA-BMImCl-Li 2 SO 4The gel polymer electrolyte was freeze-dried for 72 hours, and the morphology of GPE was characterized using a Hitachi, S-4800 scanning electron microscope (SEM).
[0056] figure 2 for different Li 2 SO 4 ContentPVA-BMImCl-Li 2 SO 4 Electron micrograph of the gel polymer electrolyte, when Li 2 SO 4 When the content is higher than 38.5wt%, the interior of the GPE has Li 2 SO 4 Crystallized out.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Specific capacitance | aaaaa | aaaaa |
| Specific capacitance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com