Prophylactic or therapeutic drug for constipation
A constipation and pharmaceutical technology, applied in the direction of drug combination, pharmaceutical formula, preparation for in vivo test, etc., can solve the problem of unknown constipation prevention or treatment effect, etc., and achieve the effect of stable effect, chemical stability and less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
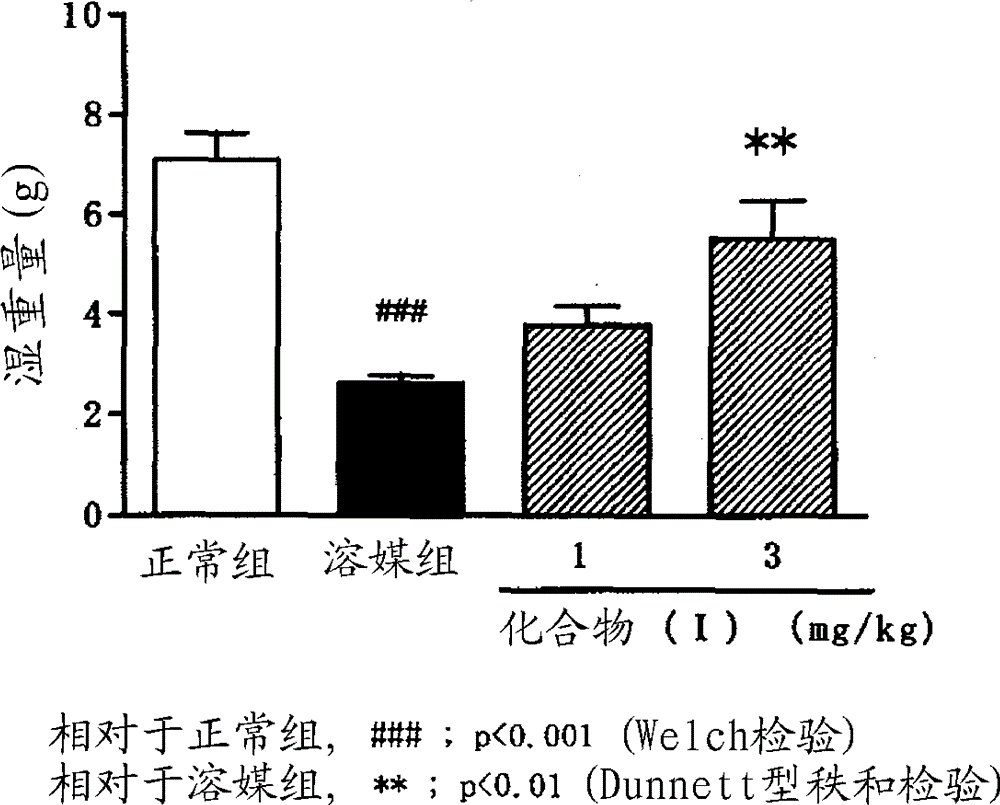
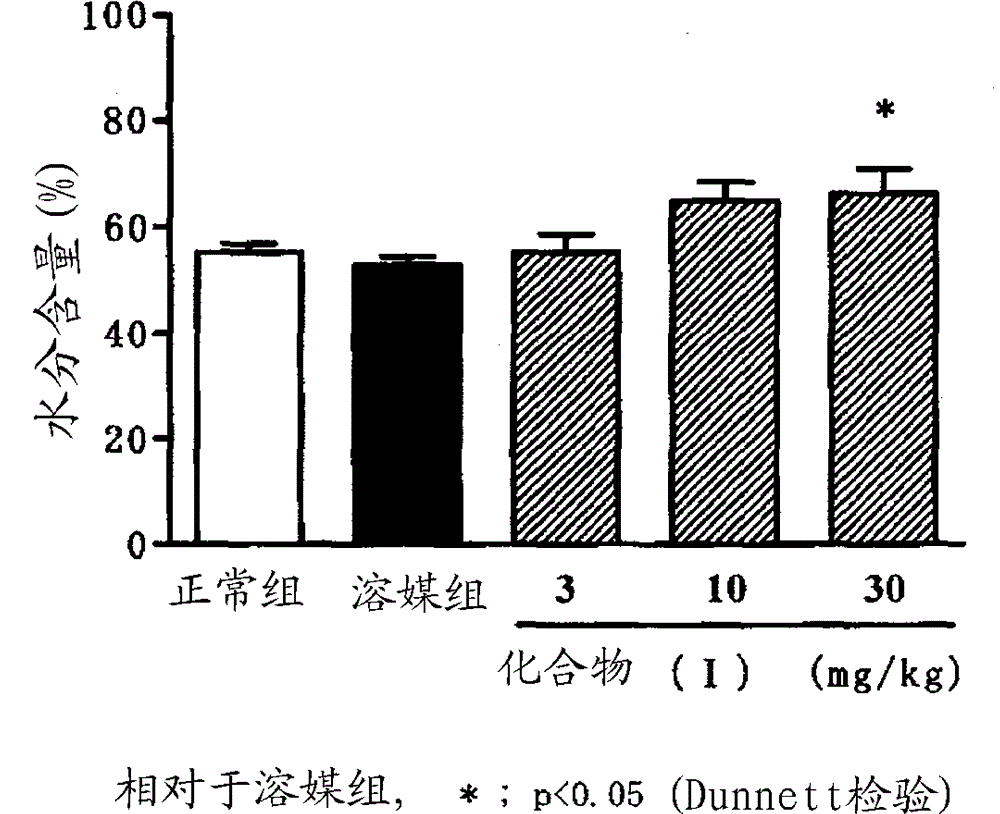
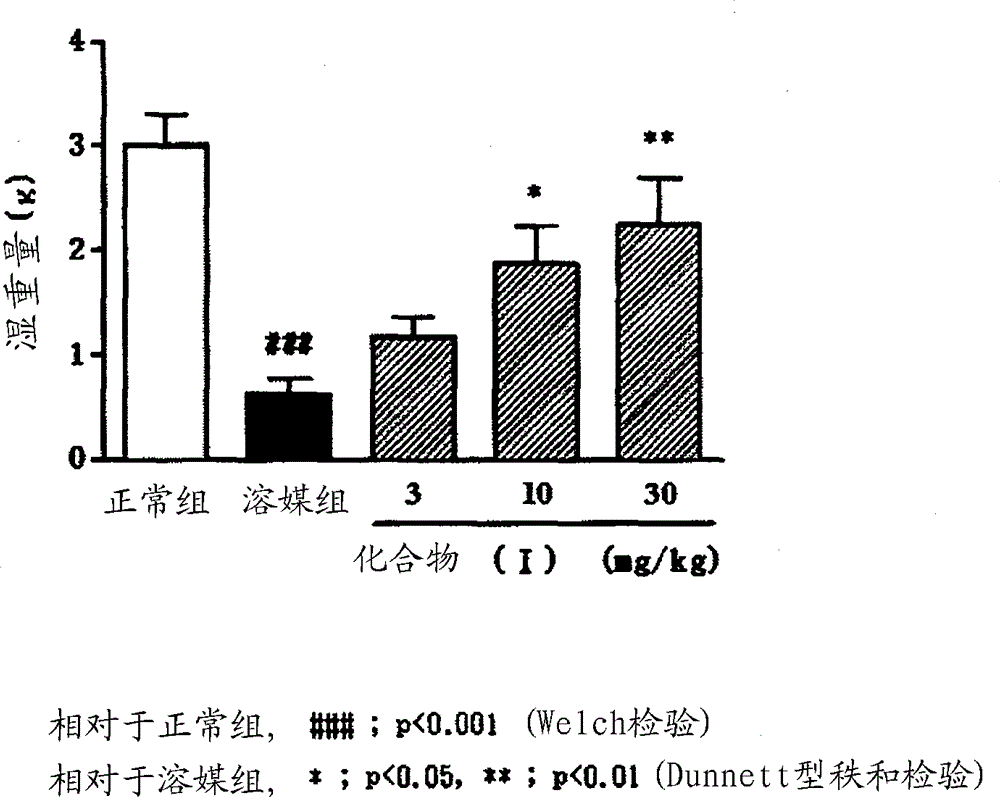
Image
Examples
reference example 1
[0131] Manufacture of compound (I)
[0132] Step 1: Fabrication of compound 3
[0133] In a solution of compound 1 (95.0g, 281mmol) in tetrahydrofuran (1395mL), under an argon atmosphere, a n-butyllithium hexane solution (2.66M, 111mL) was added dropwise at -75 to -72°C for 45 minutes. Stir at 75~-72°C for 33 minutes. Next, a solution of compound 2 (138 g, 295 mmol) in tetrahydrofuran (400 mL) was added dropwise at -78 to -73°C over 1 hour and 37 minutes, and stirred at -76 to -73°C for 20 minutes. Next, trimethylchlorosilane (32.1 g, 295 mmol) was added dropwise at -76 to -73°C over 5 minutes, followed by stirring at -76 to -73°C for 1 hour and 15 minutes. Next, n-butyllithium hexane solution (2.66 M, 153 mL) was added dropwise at -76 to -72°C over 1 hour and 5 minutes, followed by stirring at -76 to -72°C for 20 minutes. Next, a solution of 4-bromobenzaldehyde (57.2 g, 309 mmol) in tetrahydrofuran (400 mL) was added dropwise at -75 to -72°C over 1 hour and 5 minutes, foll...
reference example 2
[0139] Step 2: Fabrication of Compound 4
[0140] Acetic anhydride (12.8 mL) was added to a solution of compound 3 (8.92 g) in pyridine (30.0 mL), followed by stirring at 22 to 27° C. for 23 hours and 40 minutes. The reaction solution was cooled in an ice-water bath, water (30.0 mL) was added, and after stirring for 10 minutes, toluene (50.0 mL) was added. After separating into an organic layer and an aqueous layer, the aqueous layer was extracted with toluene (50.0 mL), and separated into an organic layer and an aqueous layer. The combined organic layers were washed three times with 2M hydrochloric acid (50.0 mL), followed by saturated aqueous sodium bicarbonate (50.0 mL) and saturated aqueous sodium chloride (50.0 mL), and separated into an organic layer and an aqueous layer. After the organic layer was dried over anhydrous magnesium sulfate (7.02 g), the solvent was distilled off under reduced pressure, followed by drying under reduced pressure to obtain a crude product (1...
reference example 3
[0148] Step 3: Fabrication of compound 5
[0149] Tert-butyldimethylsilane (6.07 g) and water (0.235 mL) were added to a solution of compound 4 (9.62 g) in acetonitrile (96.0 mL), and cooled with an ice-water bath. Trimethylsilyl trifluoromethanesulfonate (10.1 mL) was added dropwise to the mixture over 13 minutes at 1 to 7°C, followed by stirring at 5 to 11°C for 1 hour and 15 minutes. Toluene (100 mL) was added to the reaction liquid, and the mixture was washed twice with 3% aqueous sodium bicarbonate (50.0 mL). After the organic layer was distilled off under reduced pressure, the residue was dried under reduced pressure to obtain a colorless amorphous crude product (9.29 g). The crude product was treated with silica gel column chromatography [using hexane: ethyl acetate = 2:1, 1:1 (v / v), ethyl acetate to elute sequentially] to obtain compound 5 (1.12g) as a colorless powder .
[0150]
[0151]
[0152]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com