Preparation method and applications of ferriferrous oxide-polyaniline-gold nano composite material
A technology of nanocomposite material and ferric tetroxide, applied in the field of preparation of nanocomposite materials, can solve the problems of operating cost, reaction conditions, high equipment requirements, ineffective degradation, slow degradation speed, etc. The effect of reducing operating costs and fast degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Step 1: Preparation of Fe with a particle size of 200-350 nm by solvothermal method 3 o 4 Nanoparticles, the steps are as follows: 1gFeCl 3 ·6H 2 O and 0.4g of trisodium citrate were added to 30mL of ethylene glycol, after the dissolution was complete, 2.4g of anhydrous sodium acetate was added under stirring, and after stirring for 30min, the mixed solution was transferred to a 50mL polytetrafluoroethylene autoclave. The reactor was reacted in a thermostat at 200° C. for 9 h. After the reaction, cool naturally to room temperature and separate with a magnet to obtain black Fe 3 o 4 Nanoparticles were washed 3 times with distilled water and absolute ethanol respectively, and dried in vacuum for later use;
[0036] Step 2: Preparation of Fe 3 o 4 -PANI core-shell structure composite material: take the Fe prepared in step 1 3 o 4 Dissolve 0.2 g of nanoparticles in 200 mL of water, stir evenly, add 0.2 g of PVP, stir and sonicate for 30 minutes to prepare a mixed s...
Embodiment 2
[0045] Compared with Example 1, the difference is only that step 2 prepares Fe 3 o 4 -PANI core-shell structure composite material, namely: take the Fe that step 1 makes 3 o 4 Dissolve 0.2 g of nanoparticles in 200 mL of water, stir evenly, add 0.2 g of PVP, stir and sonicate for 30 minutes, and stir the mixed solution at room temperature for 3 hours. Then add 0.2g of aniline monomer and 1g of citric acid in sequence, stir and sonicate for 15min. After stirring for 10 minutes in an ice bath, 0.5 g of ammonium peroxodisulfate (APS) was added, stirred for 9 hours in an ice bath, and the product was repeatedly washed with distilled water and absolute ethanol.
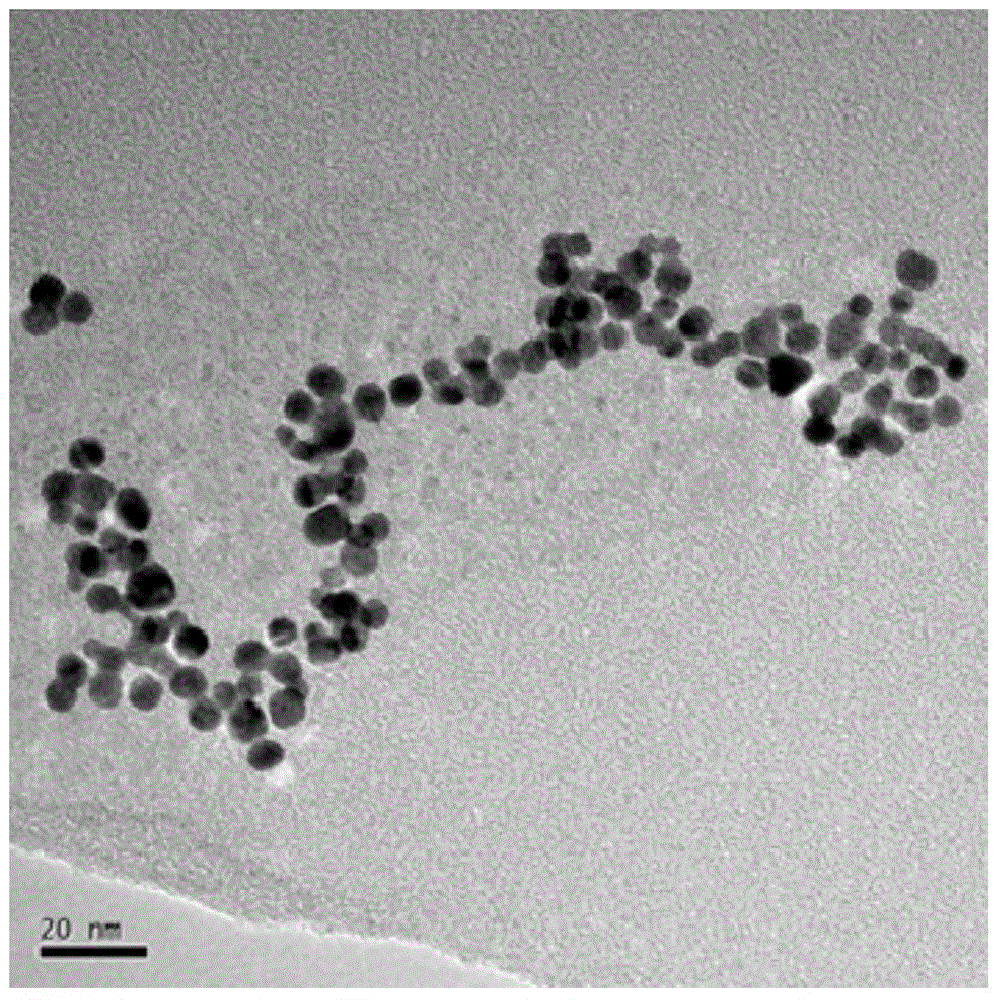
[0046] Because, compared with embodiment 1, embodiment 2 increases the amount of aniline monomer, so that the polyaniline layer coated on the surface of ferric oxide becomes thicker, which can be compared Figure 4 and Figure 5 Fe 3 o 4 -The partial enlarged figure of PANI draws, can obviously find out Fe in embodi...
Embodiment 3
[0048] Compared with Example 1, the only difference is that in Step 8, Step 7 is repeated 3 times.
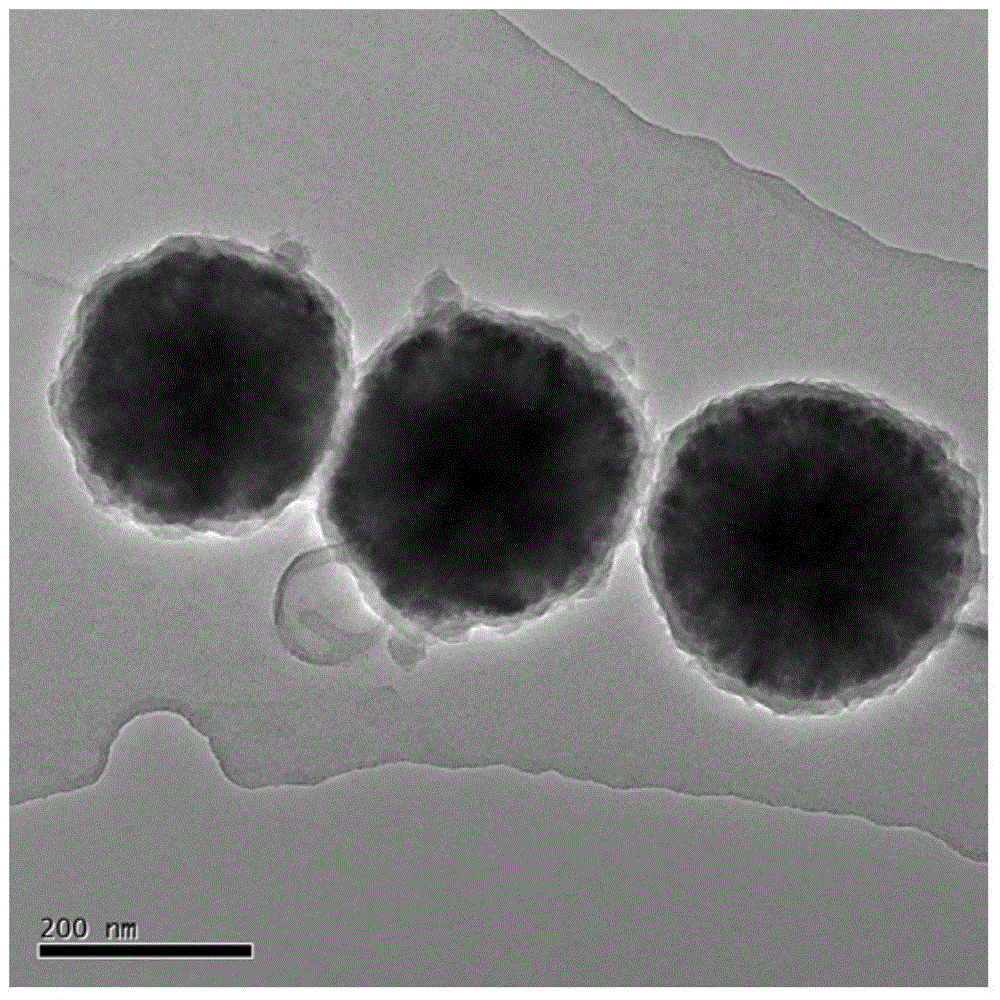
[0049] Compared with Example 1, Example 3 increased the number of repetitions of step 7, so that the reduction reaction occurred many times, so the final obtained Fe 3 o 4 -PANI-Au II The loading of gold on the surface of the nanocomposite increases, and the gold shell becomes denser. Figure 8 and Figure 7 The comparison chart is consistent with the results.
[0050] Figure 9 It is the UV-vis figure of the catalyzed p-nitrophenol obtained in Example 3 of the present invention. It can be seen from the figure that the composite material prepared by the present invention has the characteristics of sensitivity and rapidity in catalyzing p-nitrophenol.
[0051] Figure 10 For the Fe that the embodiment of the present invention 3 obtains 3 o 4 -PANI-Au II (black) and Fe 3 o 4 -PANI-Au I (twill) Reuse times map. The figure illustrates that encapsulating the gold shell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com