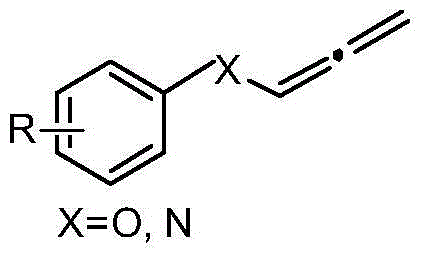
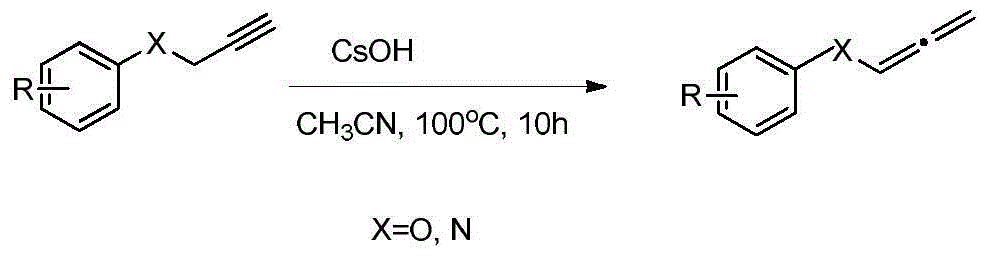Novel method for preparing allene by cesium hydroxide catalysis
A cesium hydroxide and allene technology, applied in the field of green catalytic synthesis, achieves the effects of wide source of raw materials, high reaction selectivity and yield, and simple experimental operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0017] P-nitrophenyl propargyl ether was added to a 10 mL reaction tube, cesium hydroxide (0.2 eq) was added, and 1 mL of acetonitrile was added dropwise, and the reaction was carried out at 20° C. for 0.5 h. After the reaction, the target compound was separated by column chromatography to obtain a yellow solid with a yield of 97%.
preparation example 2
[0019] Phenyl propargyl ether was added to a 10 mL reaction tube, cesium hydroxide (0.4 eq) was added, 1 mL of tetrahydrofuran was added dropwise, and the reaction was carried out at 40° C. for 1 h. After the reaction, the target compound was separated by column chromatography to obtain a colorless liquid with a yield of 94%.
preparation example 3
[0021] P-methoxyphenyl propargyl ether was added to a 10 mL reaction tube, cesium hydroxide (0.2 eq) was added, and 1 mL of acetonitrile was added dropwise, and the reaction was carried out at 30° C. for 3 h. After the reaction, the target compound was separated by column chromatography to obtain a colorless liquid with a yield of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com