Preparation method of stannic oxide nanocrystalline loaded tin disulfide nanosheet composite nanomaterial
A nanocrystalline composite and tin disulfide technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problems of difficulty in controlling the degree of sulfidation, easy aggregation of nanoparticles, etc., and achieve improved photocatalysis Reactivity, avoiding agglomeration, effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method of tin disulfide nanosheet loaded tin dioxide nanocrystal composite nanomaterial, comprising the following steps:
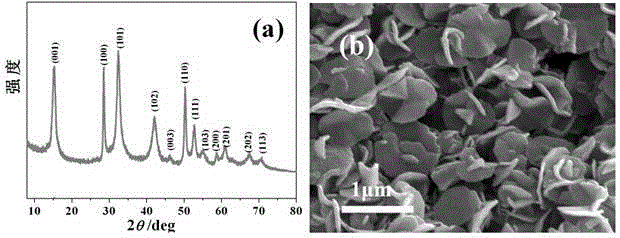
[0032] S1. Preparation of tin disulfide nanosheets: 9mmol L-cysteine and 1.5mmol tin tetrachloride are dissolved in 60ml deionized water under stirring to form a clear solution, and the molar concentration of tin ions is 0.025mol / L; The above solution was transferred to a reaction kettle, heated under hydrothermal conditions at 180°C for 10 hours, then cooled naturally, and the resulting light yellow precipitate was rinsed three times with deionized water and absolute ethanol, centrifuged and vacuum-dried to obtain the product tin disulfide SnS 2 Nanosheets.
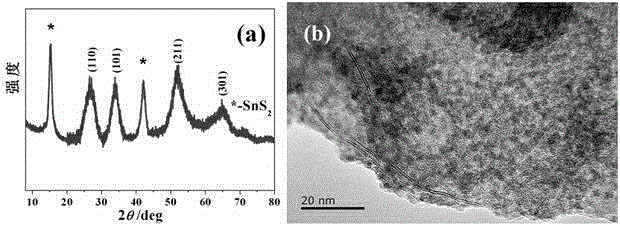
[0033] S2. Preparation of composite material: Dissolve 2mmol tin tetrachloride and 2mmol vitamin C in 60ml deionized water under stirring to form a clear solution, the molar concentration of tin ions is 0.033mol / L; Weigh the SnS prepared above as 4.85 2 nanosheets and ultras...
Embodiment 2
[0037] A preparation method of tin disulfide nanosheet loaded tin dioxide nanocrystal composite nanomaterial, comprising the following steps:
[0038] S1. Preparation of tin disulfide nanosheets: 24mmol L-cysteine and 3mmol tin tetrachloride are dissolved in 60ml deionized water under stirring to form a clear solution, and the molar concentration of tin ions is 0.05mol / L; The solution was transferred to a reaction kettle, heated under hydrothermal conditions at 180°C for 10 hours, and then cooled naturally. The resulting light yellow precipitate was rinsed three times with deionized water and absolute ethanol, separated by centrifugation and vacuum-dried to obtain the product tin disulfide SnS 2 Nanosheets.
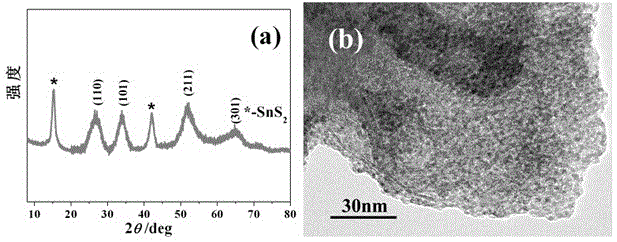
[0039] S2. Preparation of composite material: Dissolve 3mmol tin tetrachloride and 3mmol vitamin C in 60ml deionized water under stirring to form a clear solution, the molar concentration of tin ions is 0.05mol / L; Weigh the SnS prepared above as 1.21 2 nanosheets and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com