Methylprednisolone intermediate, preparation method therefor and application thereof
A technology for methylprednisolone and intermediates, which is applied in the field of methylprednisolone intermediates and its preparation, can solve the problems of serious environmental pollution, difficult treatment, high cost, etc., and achieve simple preparation process and high product purity , The effect of stable yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
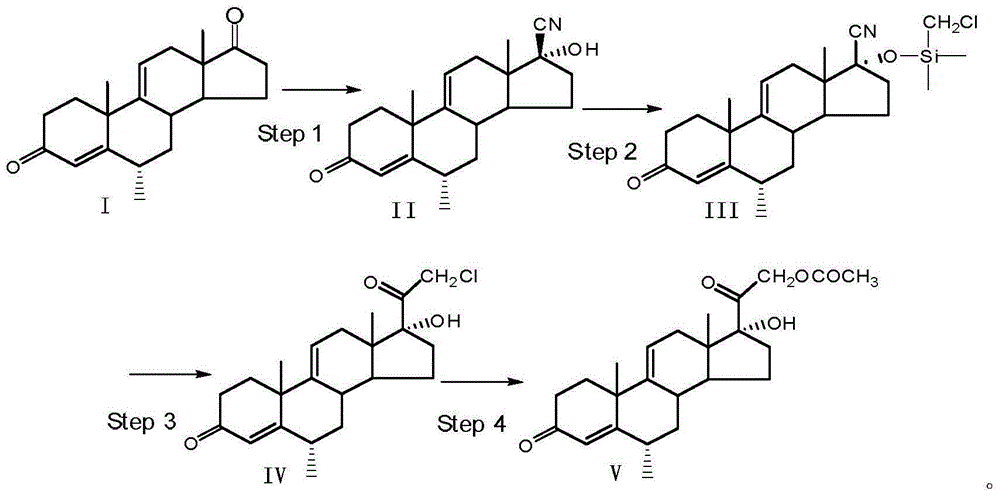
[0036] (1) Using 6αmethyl-pregna-4,9(11)-diene-3,17-dione (compound Ⅰ) as raw material, adding acetone cyanohydrin for cyano substitution reaction to prepare 6αmethyl-17βcyanide base, 17a-hydroxypregna-4,9(11)-dien-3-one (compound Ⅱ); the specific preparation method is:
[0037] 1.1 Reaction: At room temperature, under the protection of nitrogen, add 40ml of methanol, 10ml of acetone cyanohydrin with a concentration of 99%, and 20.0g of the compound to a clean and dry 250ml four-neck round-bottomed flask equipped with a thermometer, a reflux condenser, and a stirrer. 1. After stirring evenly, add 15ml of potassium carbonate aqueous solution with a mass concentration of 10%. After reacting the four-neck round bottom flask at 40-45° C. for 20 hours, it was detected by TLC that the raw materials were no longer reduced, and the reaction was stopped.
[0038] 1.2 Suction filtration: Add the reaction system in the four-neck round bottom flask dropwise to 400ml of water, and stir fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com