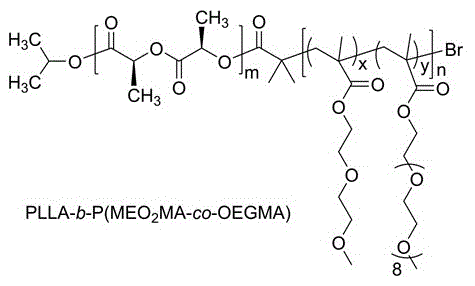
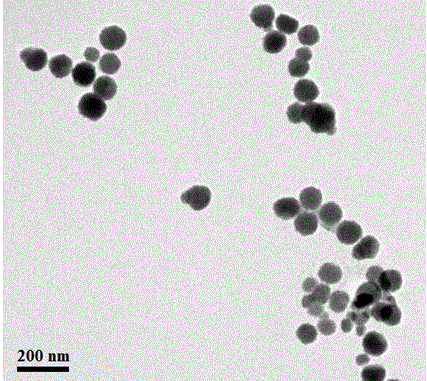
Method for preparing temperature-sensitive stereocomplex polylactic acid copolymer drug-loaded micell
A compound polylactic acid, temperature-responsive technology, applied in the fields of polymer materials and biomedical engineering, can solve problems such as side effects, and achieve the effect of improving sustained release effect and drug loading rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]Take 0.12g of isopropanol and 5.76g of L-lactide, put them in a flask, add 0.016g of stannous octoate, protect with argon, react at 130°C for 24 hours, dissolve the product with dichloromethane, and precipitate in ether 2~3 times, filter, and vacuum dry the filter cake to obtain PLLA; take 0.12g of isopropanol and 5.76g of D-lactide, put them in a flask, add 0.016g of stannous octoate, protect with argon, at 130°C React for 24 hours, dissolve the product with dichloromethane, precipitate in ether 2 to 3 times, filter, and dry the filter cake in vacuum to obtain PDLA; take 4.8g PLLA or PDLA and dissolve it in 30mL dry chloroform, under the protection of argon, to 0.0971 g of triethylamine was added to the above solution. The system was cooled to 0°C with an ice bath under constant stirring, and 0.166 g of 2-bromoisobutyryl bromide dissolved in 20 mL of dry chloroform was added dropwise to the above system within 30 min. The whole reaction system was stirred at room tempe...
Embodiment 2
[0025] Take 0.12g of isopropanol and 11.52g of L-lactide, put them in a flask, add 0.032g of stannous octoate, protect with argon, react at 130°C for 24 hours, dissolve the product with dichloromethane, and precipitate in ether 2~3 times, filter, and vacuum-dry the filter cake to obtain PLLA; take 0.12g of isopropanol and 11.52g of D-lactide, put them in a flask, add 0.032g of stannous octoate, protect with argon, at 130°C React for 24 hours, dissolve the product with dichloromethane, precipitate in ether 2 to 3 times, filter, and dry the filter cake in vacuum to obtain PDLA; take 4.8g PLLA or PDLA and dissolve it in 30mL dry chloroform, under the protection of argon, to 0.0971 g of triethylamine was added to the above solution. The system was cooled to 0°C with an ice bath under constant stirring, and 0.083 g of 2-bromoisobutyryl bromide dissolved in 20 mL of dry chloroform was added dropwise to the above system within 30 min. The whole reaction system was stirred at room te...
Embodiment 3
[0027] Take 0.17g of isopropanol and 8.06g of L-lactide, put them in a flask, add 0.022g of stannous octoate, protect with argon, react at 130°C for 24 hours, dissolve the product with dichloromethane, and precipitate in ether 2~3 times, filter, and vacuum dry the filter cake to obtain PLLA; take 0.17g of isopropanol and 8.06g of D-lactide, put them in a flask, add 0.022g of stannous octoate, protect with argon, at 130°C React for 24 hours, dissolve the product with dichloromethane, precipitate in ether for 2 to 3 times, filter, and dry the filter cake in vacuum to obtain PDLA; dissolve 6.72g PLLA or PDLA in 30mL dry chloroform, and pour to Add 0.1360g triethylamine in the above solution. The system was cooled to 0°C with an ice bath under constant stirring, and 0.232 g of 2-bromoisobutyryl bromide dissolved in 25 mL of dry chloroform was added dropwise to the above system within 30 min. The whole reaction system was stirred at room temperature under the protection of argon f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com