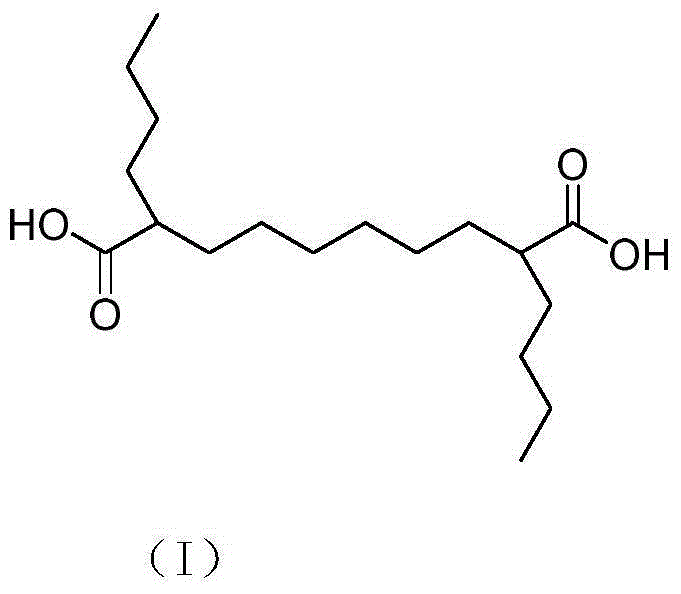
Preparation method for 2,9-dibutyl sebacate
A technology of butyl sebacic acid and dimethyl n-butyl malonate, applied in the chemical field, can solve the problems of principle toxicity, difficult separation, complicated process, etc., and achieves good product color, high reaction yield and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 94 g (0.5 mol) of commercially available butyl dimethyl malonate and 150 mL of anhydrous diethyl ether into a three-neck flask equipped with a thermometer, a condenser, and a drying tube, and start stirring. Cut 11.5g (0.5mol) of the weighed sodium block into relatively small sodium particles in n-hexane, add it to the above-mentioned reaction solution in batches within two hours, and continue to heat and reflux for 2 hours until the reaction system The sodium particles in it disappear. Then, while heating and stirring, slowly add 1,6-dibromohexane 122g (0.5mol) 150mL anhydrous ether solution dropwise to the reaction solution, control the rate of addition, so that the 1,6-dibromohexane solution is in 3 The addition was complete within hours and the reaction was heated to reflux overnight.
[0027] Heating was stopped, and the reaction system was cooled to room temperature. Add appropriate amount of dilute hydrochloric acid to the system at room temperature to make...
Embodiment 2
[0029] 91.7 g (0.2 mol) of the above-mentioned tetraester and 250 mL of methanol were successively added into a 1 L three-necked flask equipped with a thermometer and a condenser, and stirring was started, and 34 g (0.6 mol) of potassium hydroxide solution was added. The oil bath was heated to reflux, and the heating was stopped after 14 hours, and the reaction system was cooled to room temperature. Add 50mL of concentrated hydrochloric acid dropwise to the system under an ice-water bath until the solution is acidic. At this time, a large amount of white solids will be found to separate out. Filter and wash the filter cake with water to neutrality, then dry. The dried solid product is 71.6g. The rate is 89%.
Embodiment 3
[0031] Return the solid 8.04g (20mmol) obtained in Example 2 to the reactor, heat up while stirring, and when the temperature is raised to partially dissolve the solid, maintain this temperature and stir to completely liquefy the solid, and continue decarboxylation at this temperature React until the reaction is essentially complete (approximately 4-5 hours are required). Cool to room temperature. After dissolving in ethyl acetate, pure ethyl acetate was used as eluent to separate by column chromatography to obtain pure 2,9-dibutyl sebacic acid as a white solid, weighing 5.72 g, with a yield of 91%.
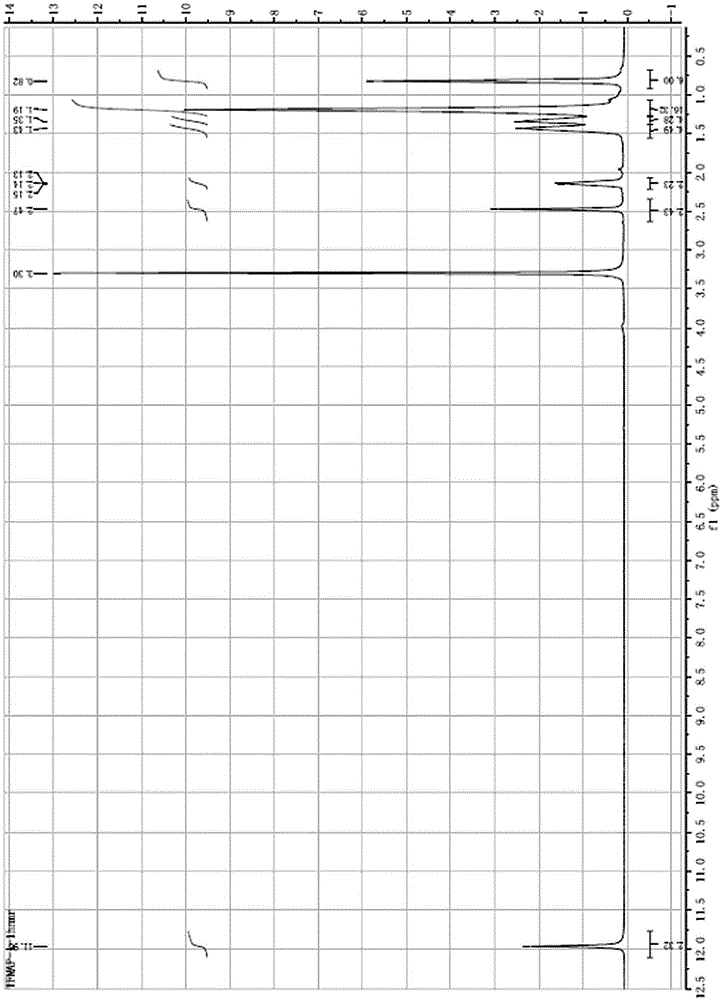
[0032] 1 HNMR (400MHz, d- DMSO): δ0.82(m,6H),1.19(m,16H),1.35(m,4H),1.43(m,4H),2.13-2.150.93(t,J=4Hz,2H),11.9(s ,2H).
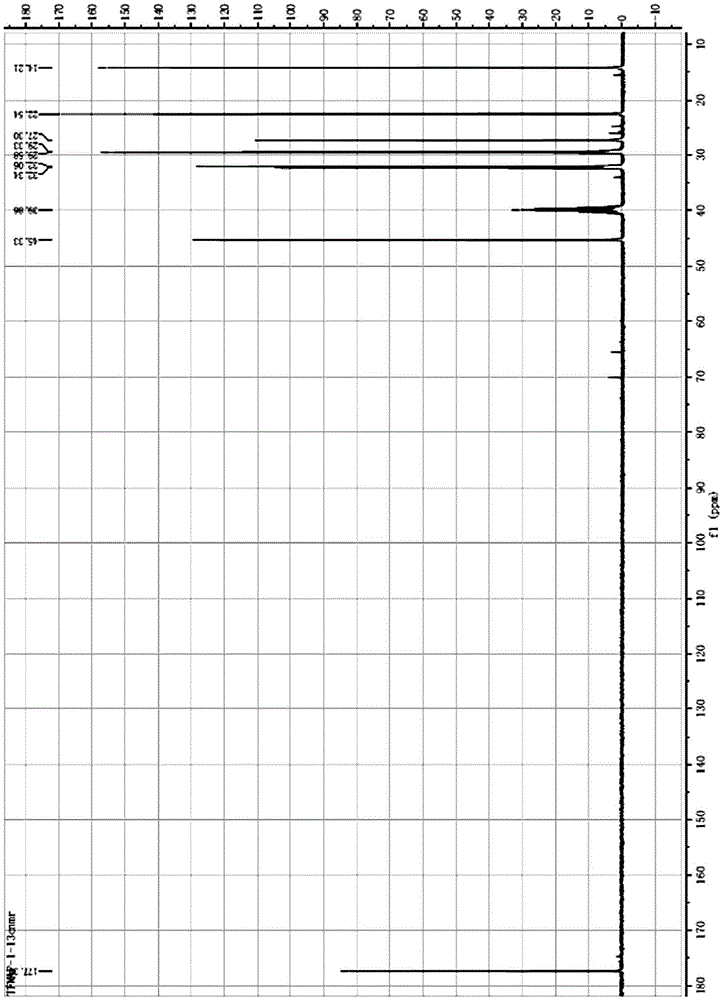
[0033] 13 CNMR (400MHz, d- DMSO): δ177.39, 45.33, 32.34, 32.06, 29.58, 29.33, 27.30, 22.54, 14.21
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com