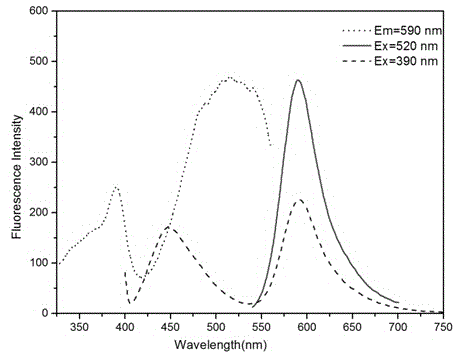
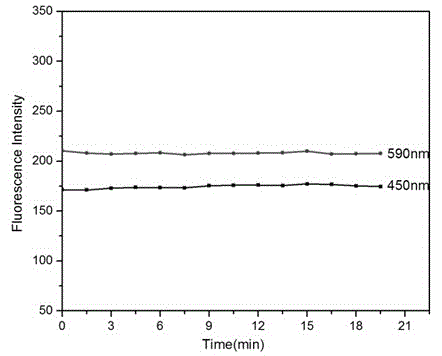
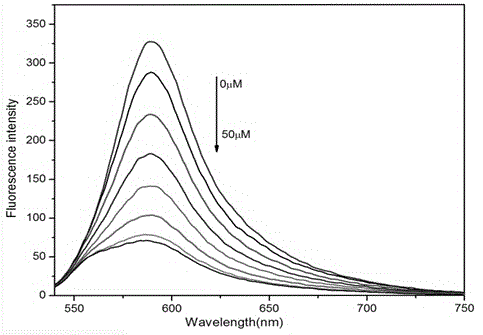
Visible and reversible ratiometric fluorescent probe as well as preparation method and application thereof
A ratiometric fluorescent probe and reaction technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve the effect of reducing pollution, saving resources, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of ratiometric fluorescent probe 5-1 (R is propanesulfonic acid), the structural formula is as follows
[0046]
[0047] Take 5mL of salicylaldehyde and 2.35g of paraformaldehyde in a 250mL round-bottomed flask containing 20mL of concentrated hydrochloric acid. After the mixture is evenly mixed, start adding 1.5mL of POCl dropwise. 3 , added within 1 hour, the mixture was reacted at room temperature for 18 hours, and the precipitate was filtered out and washed with 3% NaHCO 3 After washing with ultrapure water three times respectively, put it into a vacuum drying oven to dry, then recrystallize with petroleum ether to obtain product 1, mass spectrometry ESI-MS: m / z (%): 169.59 (M - -1).
[0048] Add 12.5mmol of product 1 and 16.2mmol of cyclohexamethylenetetramine to 13mL of 50% acetic acid solution, heat to 120°C, reflux the reaction solution, add 6mL of concentrated hydrochloric acid after 1 hour, the solution turns yellow, continue After stirring for...
Embodiment 2
[0056] Preparation of ratiometric fluorescent probe 5-2 (R is 3-bromopropionic acid), the structural formula is as follows
[0057]
[0058] Take 5mL of salicylaldehyde and 2.35g of paraformaldehyde in a 250mL round-bottomed flask containing 20mL of concentrated hydrochloric acid. After the mixture is evenly mixed, start adding 1.5mL of POCl dropwise. 3 , added within 1 hour, the mixture was reacted at room temperature for 18 hours, and the precipitate was filtered out and washed with 3% NaHCO 3 and ultrapure water for three times, put it in a vacuum oven for drying, and recrystallize with petroleum ether to obtain product 1.
[0059] Add 12.5mmol of product 1 and 16.2mmol of cyclohexamethylenetetramine to 13mL of 50% acetic acid solution, heat to 120°C, reflux the reaction solution, add 6mL of concentrated hydrochloric acid after 1 hour, the solution turns yellow, continue After stirring for 5 minutes in an ice-water bath, the crude product precipitated out, and the light...
Embodiment 3
[0066] Preparation of ratiometric fluorescent probe 5-3 (R is bromoacetic acid), the structural formula is as follows
[0067]
[0068] Take 5mL of salicylaldehyde and 2.35g of paraformaldehyde in a 250mL round-bottomed flask containing 20mL of concentrated hydrochloric acid. After the mixture is evenly mixed, start adding 1.5mL of POCl dropwise. 3 , added within 1 hour, the mixture was reacted at room temperature for 18 hours, and the precipitate was filtered out and washed with 3% NaHCO 3 and ultrapure water for three times, put it in a vacuum oven for drying, and recrystallize with petroleum ether to obtain the product 1.
[0069] Add 12.5mmol of product 1 and 16.2mmol of cyclohexamethylenetetramine to 13mL of 50% acetic acid solution, heat to 120°C, reflux the reaction solution, add 6mL of concentrated hydrochloric acid after 1 hour, the solution turns yellow, continue After stirring for 5 minutes in an ice-water bath, the crude product precipitated out, and the light ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com