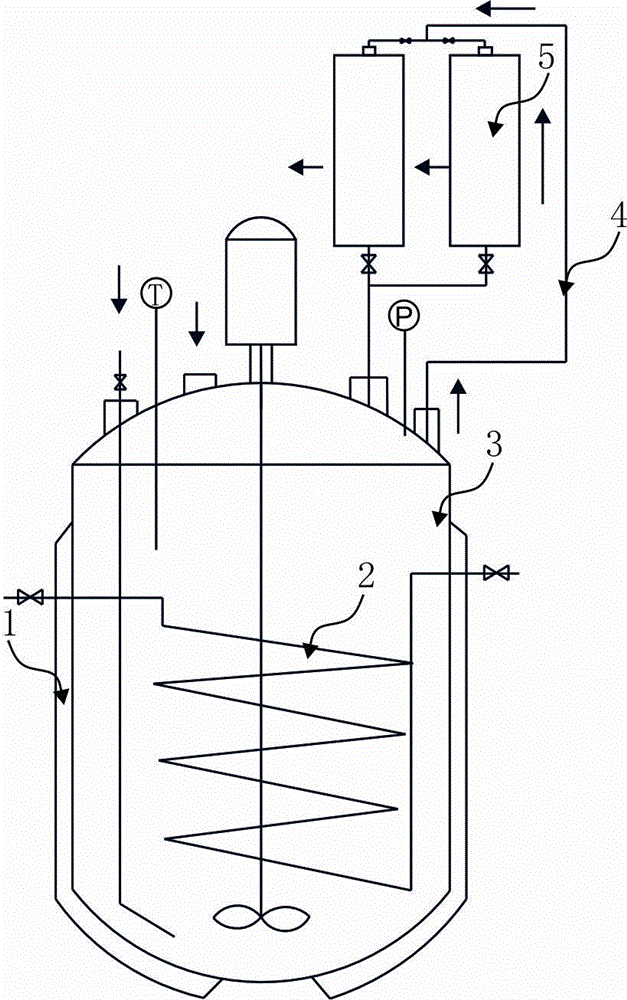
Process for synthesis of 3-hexyne- 2, 5-diol by slurry bed acidity control method
An acidity control, slurry bed technology, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problems of unsuitability for industrial production, large market gap, low safety, etc., and reduce consumption costs. , The effect of reducing production cost and high production rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation method of 3-hexyne-2,5-diol is as follows:
[0044] (1) Preparation of catalyst: Dissolve 75g of copper nitrate in 600ml of soft water at 60°C and stir to dissolve; dissolve 13.5g of bismuth nitrate in 180ml of soft water at 80°C and stir to dissolve; dissolve 75g of sodium carbonate in Stir to dissolve in 750ml soft water at 60°C; slowly add copper nitrate and bismuth nitrate solutions to the sodium carbonate solution at the same time, continue stirring for 20-25 minutes, and then adjust the pH value of the solution with nitric acid with a mass fraction of 65% to 7~8, heat-preserve and react at 50°C for 4 hours to obtain a product liquid, vacuum filter the prepared liquid, rinse the filter cake repeatedly with soft water at 60°C until neutral, and place the obtained filter cake in Dry in a constant temperature drying oven at 95°C for 5 hours to obtain 42.6g of a catalyst, which is a mixture of basic copper carbonate and bismuth basic carbonate. The prepar...
Embodiment 2
[0053] The preparation method of 3-hexyne-2,5-diol is as follows:
[0054] (1) The preparation and activation of the catalyst are the same as the preparation and activation of the catalyst in Example 1.
[0055] (2) Then add 1100ml of acetaldehyde aqueous solution with a mass fraction of 45% and 80g of catalyst into the slurry bed reactor, replace the reactor with acetylene for 3 to 5 times, start stirring, and slowly raise the temperature to 120°C, and at the same time The compressed 162.5g of acetylene is imported from the lower part of the reactor, and the pressure in the reactor is controlled at 1.3Mpa to carry out the stirring reaction. After 13 hours of reaction, take samples from the sampling valve of the reactor, and take samples every half an hour until the product content is qualified. , to obtain the resulting solution;
[0056] In the reaction process, when the reaction is carried out to 5 / 3 hours, the 25ml sodium hydroxide solution with a mass concentration of 3%...
Embodiment 3
[0061] The preparation method of 3-hexyne-2,5-diol is as follows:
[0062] (1) The preparation and activation of the catalyst are the same as the preparation and activation of the catalyst in Example 1.
[0063] (2) Then add 1100ml of acetaldehyde aqueous solution with a mass fraction of 48% and 88g of catalyst into the slurry bed reactor, replace the reactor with acetylene for 3 to 5 times, start stirring, and slowly raise the temperature to 120°C, and at the same time The compressed 173g of acetylene is introduced from the lower part of the reactor, and the pressure in the reactor is controlled at 1.0Mpa to carry out the stirring reaction. After 10 hours of reaction, take samples from the reactor sampling valve, and take samples every half an hour until the product content is qualified. to obtain the resulting liquid;
[0064] In the reaction process, when the reaction is carried out to 4 / 3 hour, the 20ml sodium hydroxide solution with a mass concentration of 3.0% is pumped...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com