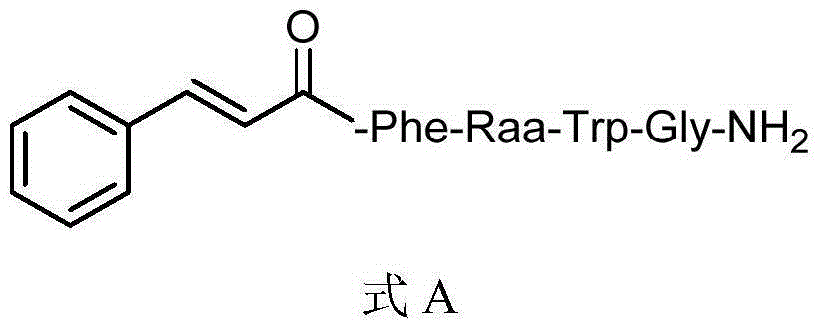
Insect kinin analogs and application thereof
A technology of insect kinin and analogs, applied in the fields of application, peptides, pesticides, etc., can solve the problems of not very prominent biological activity and large molecular weight of kinin analogs, and achieve good in vitro inhibitory activity and good control effect. , The effect of insecticidal activity is obvious
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
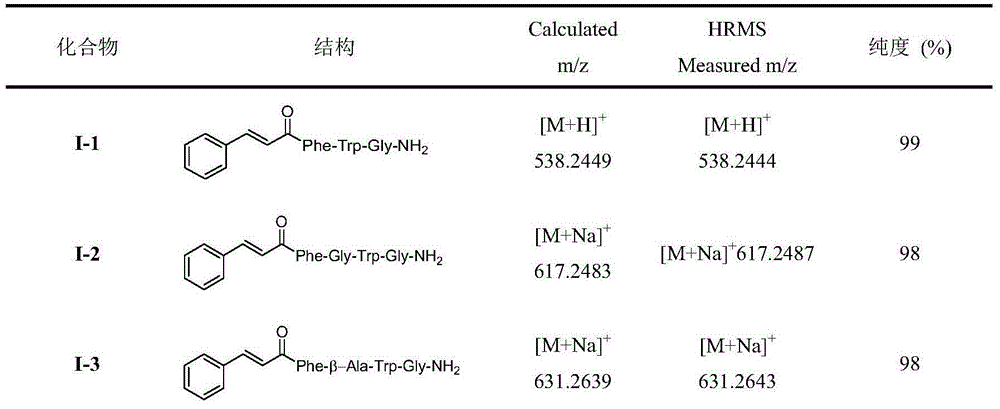
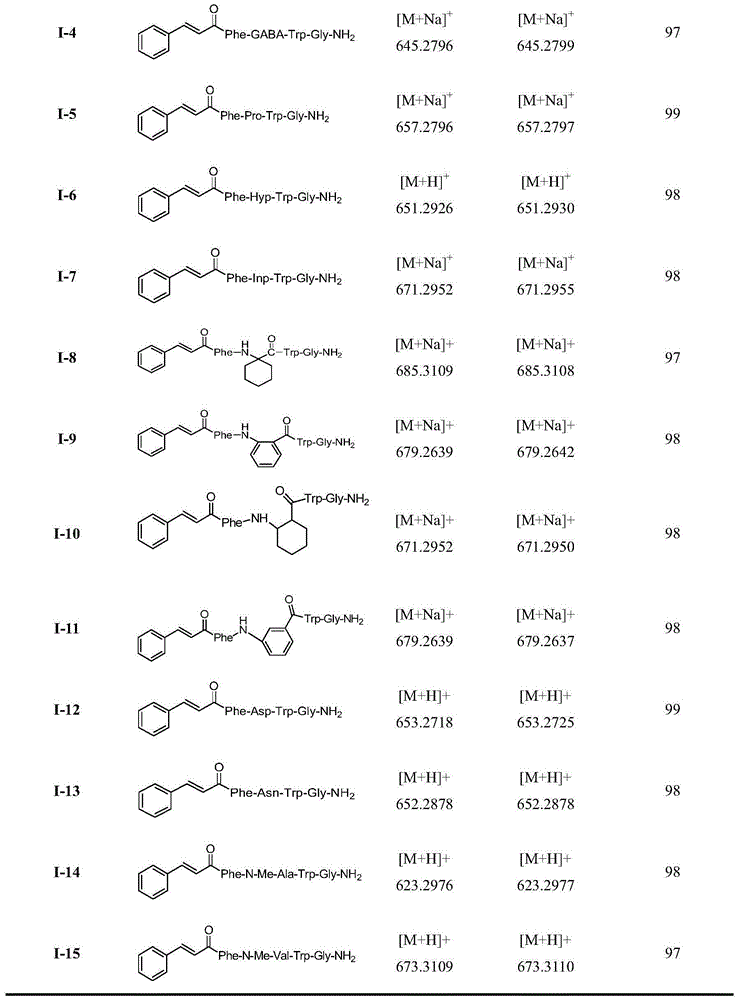
[0021] Embodiment 1: Preparation of 1-2 compound (when Raa is Gly)
[0022](1) Resin activation: Weigh 500mg RinkAmide-AMresin, wash 4 times with DCM, add 5ml DCM for swelling and activation for 3h, wash 4 times with DMF, add 20% piperidine DMF solution to cut for 20min, wash 4 times with 5ml DMF, wash 4 times with 5ml DCM, Kaiser's Reagent testing.
[0023] (2) Connect Gly: DMF wash 3 times, add 360mgFmoc-Gly-OH (4 times the amount), 455mgHBTU (4 times the amount), 162mgHOBt (4 times the amount), 142ulDIEA (4 times the amount), dissolve in 5ml DMF, stir at room temperature 2h, wash 4 times with 5ml DMF, add 20% piperidine DMF solution to cut for 20min, wash 4 times with 5ml DMF, wash 4 times with 5ml DCM, and detect with Kaiser's reagent.
[0024] (3) Receive Trp(Boc): Wash 3 times with DMF, add 630mgFmoc-Trp(Boc)-OH (4 times the amount), 455mgHBTU (4 times the amount), 162mgHOBt (4 times the amount), 142ulDIEA (4 times the amount), Dissolve in 5ml DMF, stir at room tempera...
Embodiment 2
[0034] Embodiment 2: the biological activity of formula A target compound to aphids
[0035] The insecticidal activity of the target compound of formula A to aphids is determined by the leaf-dipping method. The target compound was made into a 2000 mg / L assay solution. Then use a 1-5ml pipette gun to take 1ml of acetone and add it to a weighing bottle, add 9ml of an aqueous solution containing 0.1% Triton X-100, and mix well to obtain a 200mg / L measurement solution, and then use 0.1% Triton X-100 Dilute step by step with the aqueous solution of X-100, and mix thoroughly to obtain the required concentration. Cultivate soybean aphids and soybean leaves that have not been exposed to any pesticides indoors, use a puncher with a diameter of 15mm to punch out leaves of appropriate size, immerse them in the diluted medicinal solution for 15 seconds, and take them out to dry. Then the leaves were put into a bioassay plate, 1% agar was added to the bottom to moisturize, 20±3 soybean a...
Embodiment 3
[0042] Embodiment 3: the biological activity of formula A target compound to plant pathogenic bacteria
[0043] The indoor and in vitro inhibitory activity of the compounds against plant pathogenic bacteria was determined by the turbidity-ELISA method.
[0044] The tested target bacteria were 7 common pathogens, including Phytophthoracapsici, Fusarium fujikuroi, Bipolarismaydis, Colletotrichumgloeosporioides, Botrytiscinerea , tomato early blight (Alternaria solani) and corn head smut (Sporisorium reilianum).
[0045] Compounds to be tested were formulated with DMSO into 10 4 mg / L mother solution, and then diluted with sterile water to form a series of gradient concentration drug solutions, and placed at 4°C for later use.
[0046] Cultivation of target bacteria: Inoculate the strains that are prone to spore production (bakanae rice bacterium, pepper early blight bacterium, and corn spot bacterium) on the optimal medium plate and carry out spore production culture. After it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com