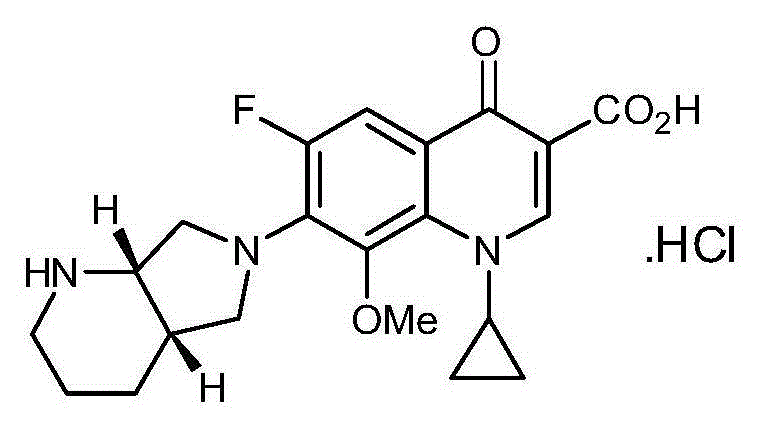
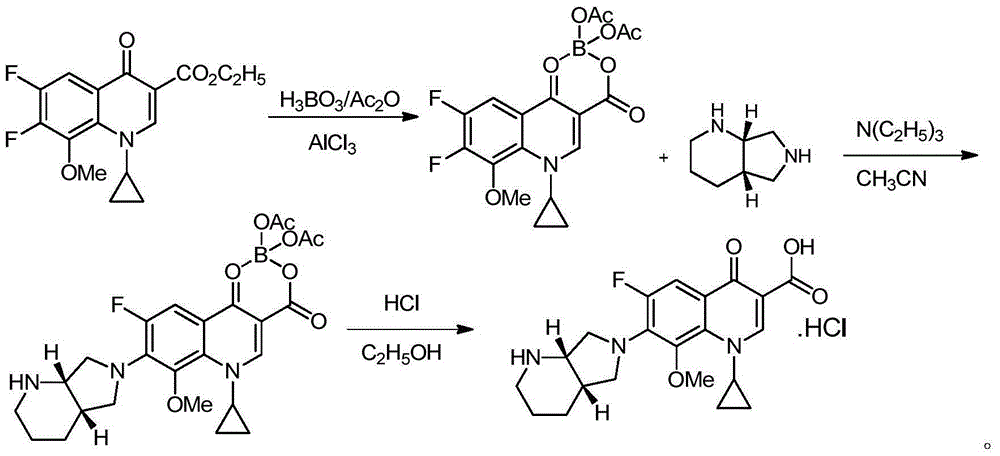
Method for preparing high-purity moxifloxacin hydrochloride
A moxifloxacin hydrochloride, high-purity technology, applied in the field of drug synthesis, can solve the problems of complicated steps, many materials, difficult to control, etc., and achieve the effect of simple operation and post-processing, energy saving, and simple route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]1-cyclopropyl-6,7-difluoro-8-methoxy-1,4-dihydro-4-oxoquinoline-3-carboxylic acid-O 3 ,O 4 -Preparation of boron diacetate
[0029] Add 93g of boric acid, 3.3g of anhydrous aluminum trichloride and 500ml of acetic anhydride into the reaction flask, stir evenly, slowly heat up to 85-90°C, stir and react for 1 hour, cool in a water bath, and lower the temperature to 50°C-60°C, Add 323.3 g of ethyl quinolinecarboxylate, and continue to stir and react at a temperature of 85° C. to 90° C. for 3 hours. Followed by HPLC until the reaction was completed (ethyl quinolinecarboxylate was reduced to below 5%), the solvent was evaporated under reduced pressure to obtain an oily substance, the oily substance was slowly added to 8.08L ice water, stirred for 30 minutes to precipitate the crude product, filtered, and the filter cake was watered After washing until neutral, the filter cake was dried under reduced pressure at 60° C. in a drying oven for 8 hours to obtain 402 g of an off-...
Embodiment 2
[0036] 1-cyclopropyl-6,7-difluoro-8-methoxy-1,4-dihydro-4-oxoquinoline-3-carboxylic acid-O 3 ,O 4 -Preparation of boron diacetate
[0037] Add 95g of boric acid, 3.3g of anhydrous aluminum trichloride and 500ml of acetic anhydride into the reaction flask, stir evenly, slowly heat up to 85-90°C, stir and react for 1 hour, cool in a water bath, and lower the temperature to 50°C-60°C, Add 323.3 g of ethyl quinolinecarboxylate, and continue to stir and react at a temperature of 85° C. to 90° C. for 3 hours. Followed by HPLC until the reaction is complete (ethyl quinolinecarboxylate is reduced to below 5%), the solvent is evaporated under reduced pressure to obtain an oily substance, the oily substance is slowly added to 8.8L ice water, stirred for 30 minutes to separate out the crude product, filtered, and the filter cake was watered After washing until neutral, the filter cake was dried under reduced pressure at 60° C. in a drying oven for 8 hours to obtain 389 g of off-white s...
Embodiment 3
[0044] 1-cyclopropyl-6,7-difluoro-8-methoxy-1,4-dihydro-4-oxoquinoline-3-carboxylic acid-O 3 ,O 4 -Preparation of boron diacetate
[0045] Add 92g of boric acid, 3.2g of anhydrous aluminum trichloride and 500ml of acetic anhydride into the reaction flask, stir evenly, slowly heat up to 85-90°C, stir and react for 1 hour, cool in a water bath, and lower the temperature to 50°C-60°C, Add 323.3 g of ethyl quinolinecarboxylate, and continue to stir and react at a temperature of 85° C. to 90° C. for 3 hours. Followed by HPLC until the reaction is complete (ethyl quinolinecarboxylate is reduced to below 5%), the solvent is evaporated under reduced pressure to obtain an oily substance, the oily substance is slowly added to 7.8L ice water, stirred for 30 minutes to separate out the crude product, filtered, and the filter cake was watered After washing until neutral, the filter cake was dried in a drying oven at 60°C under reduced pressure for 8 hours to obtain 404 g of off-white sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com