Design and application of PCSK9 targeting recombinant vaccine
An immune response and composition technology, applied in the field of PCSK9-targeted recombinant vaccine design, can solve problems such as muscle toxicity, elevation of liver transaminases, and adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Embodiment 1 Recombinant plasmid construction and fusion protein expression
[0168] 1.1 Design the fusion protein (recombinant vaccine) according to Table 2.
[0169] Table 2 Design of fusion protein
[0170]
[0171] 1.2 Plasmid construction and fusion protein expression
[0172] 1.2.1 Experimental reagents
[0173] ExTaq enzyme, 10×ExTaqbuffer, dNTPs (Takara), 10×DNA loading buffer (Takara), KOD enzyme (TOYOBO), 10×KODplusbuffer, MgSO 4 (25mmol / L), dNTPs (TOYOBO Company), Gold-view dyeing solution (SBS Race Parkson Company), DNAMarker (TIANGEN Biological Co., Ltd.).
[0174] 1.2.2 Experimental method
[0175] 1.2.2.1 Primer design and synthesis
[0176] (1) Nested primer design
[0177] Nested primers were designed according to the accession numbers of human and mouse PCSK9 gene mRNA sequences provided by GenBank (GenBank: EF692496.1, NM_153565.2), and the primers were named hF1, hR1, hF2, hR2, mF1, mR1, mF2, mR2. See Appendix 1.
[0178] (2) Fusion primer...
Embodiment 2
[0222] Example 2 Purification of Fusion Protein and Refolding of Inclusion Body
[0223] 2.1 Experimental steps
[0224] Use the conventional method to ferment the genetically engineered strain obtained in Example 1. After the fermentation is completed, it is ultrasonically crushed, and the target protein is affinity purified using a GST column. The inclusion body of PCSK9-8 is refolded and identified by SDS-PAGE electrophoresis. , BCA protein quantification method was used for protein quantification.
[0225] Concentrate the purified target protein with an ultrafiltration tube with a pore size of 3kD or 10kD, and adjust the protein concentration to 0.5-1mg / mL with 1×PBS. In the ultra-clean bench, the prepared protein solution was divided into 1.5mLEP tubes, and 15% glycerol was added, and stored in a -70°C refrigerator for later use.
[0226] 2.2 Experimental results
[0227] 2.2.1 Affinity purification of fusion protein
[0228] The size of PCSK9-1, PCSK9-2, PCSK9-3 and ...
Embodiment 3
[0239] Embodiment 3 animal immunization and titer determination
[0240] 3.1 Experimental materials and instruments
[0241] 3.1.1 Experimental reagents
[0242] mPCSK9 recombinant protein, hPCSK9 recombinant protein (purchased from Beijing Yiqiao Shenzhou Biotechnology Co., Ltd.); secondary antibody (HRP-labeled goat anti-mouse antibody), pre-stained marker, easyWesternProteinLader (purchased from GenScript Nanjing Biotechnology Co., Ltd.)
[0243] 3.2 Experimental method
[0244] 3.2.1 Mice immunization method
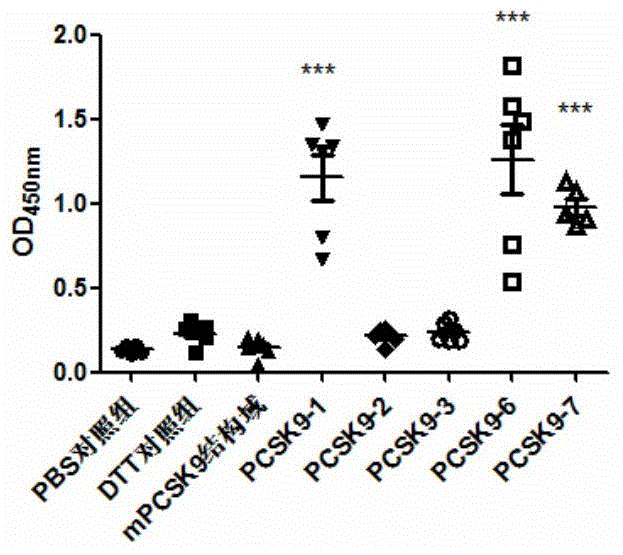
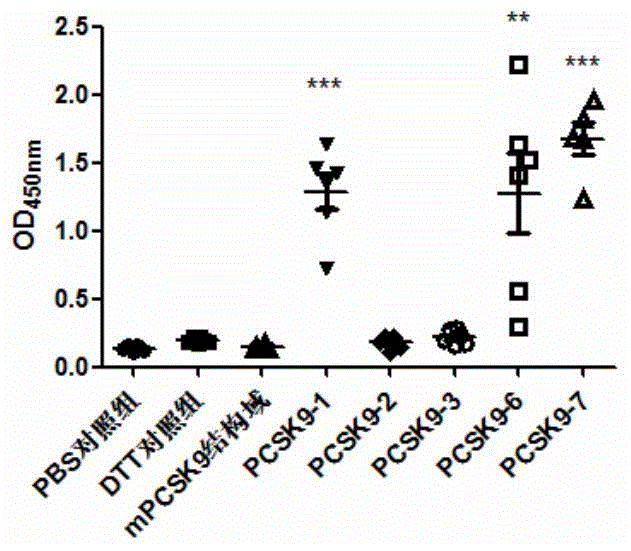
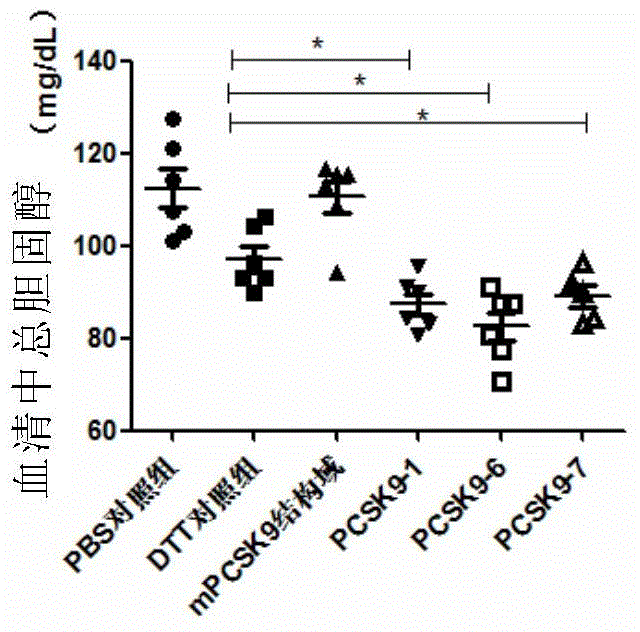
[0245] The animals used for immunization were female BALB / c mice aged 6-7 weeks at SPF level (purchased from Beijing Weitong Lihua), which were raised in the ultra-clean room of the Animal Experiment Center of Shanghai Jiaotong University. They were randomly divided into 8 groups, with 6 mice in each group. 10mMPBS, DTT, PCSK9-1, PCSK9-2, PCSK9-3, PCSK9-6, PCSK9-7 and PCSK9-8 were used as antigens respectively. Immunization was carried out on days 0, 7, and 14, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| antibody titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com