Self-supporting transition metal-phosphorus alloy catalyst, and preparation method and application thereof
A technology of alloy catalysts and transition metals, applied in the field of self-supporting transition metal-phosphorus alloy catalysts and their preparation, can solve the problems of low catalytic activity and limited pH value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The present invention also provides a preparation method of a self-supporting transition metal-phosphorous alloy catalyst, the method comprising:
[0023] Step 1: mixing metal salts of transition metal elements and phosphorus sources, and then adding surfactants or alkaline solutions to obtain electrolytes;
[0024] Step 2: Using the transition metal conductive substrate as a working electrode, performing electrodeposition in the electrolyte solution obtained in Step 1 to obtain a self-supporting transition metal-phosphorus alloy catalyst.
[0025] According to the present invention, the metal salt of the transition metal element and the phosphorus source are mixed, and then a surfactant or an alkaline solution is added, preferably dissolved in distilled water under the condition of magnetic stirring, to obtain an electrolyte; preferably, the transition metal element can be Add the molybdenum source when the metal salt and the phosphorus source are mixed, and then add t...
Embodiment 1
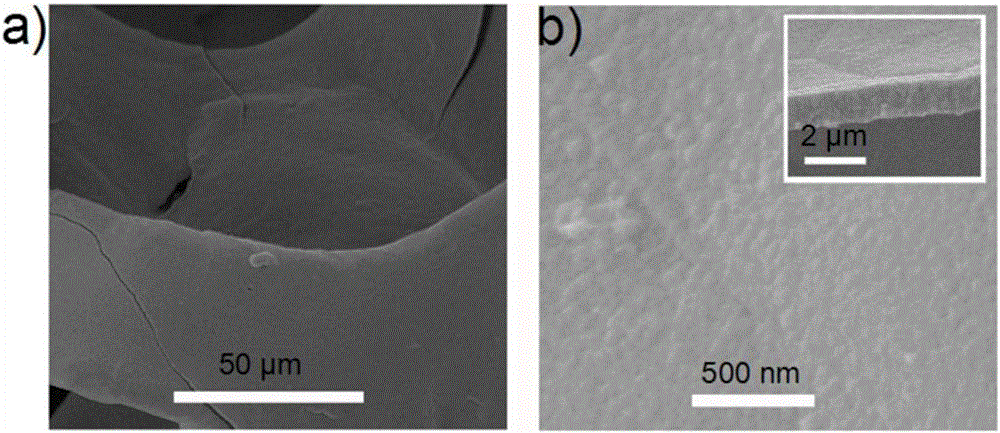
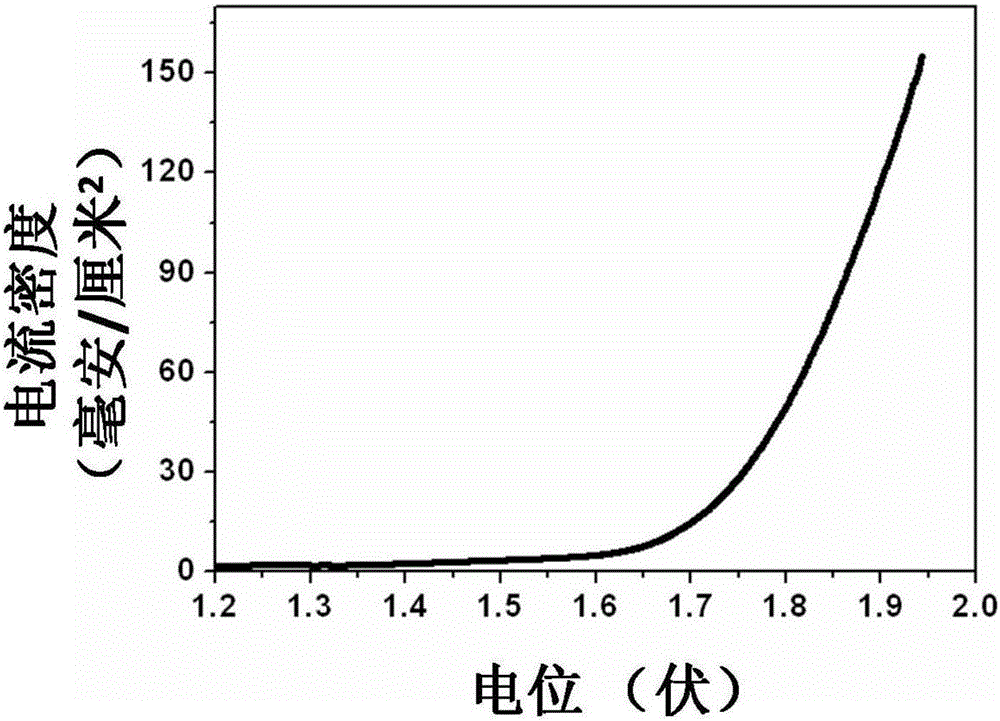
[0032] Weigh 0.1 moles of nickel sulfate, 0.3 moles of sodium hypophosphite and 0.1 moles of sodium acetate and mix them to obtain an electrolyte; wash the nickel mesh with dilute hydrochloric acid, ethanol, and deionization, then add it to the above solution, and use the nickel mesh as a working electrode , Graphite sheet is the counter electrode, saturated calomel is the reference electrode, using electrochemical workstation (CHI660D) at 25 ℃ under the condition of 0.01 A / cm 2 The constant current was reacted for 0.1 hour, and nickel-phosphorus alloy particles were obtained on the nickel mesh.
[0033] figure 1 It is the scanning electron micrograph figure of the nickel-phosphorus alloy particle that embodiment 1 prepares, and wherein figure a is the scanning electron microscope photograph under the 50 micron scale, and figure b is the scanning electron microscope photograph under the 500 nanometer scale, and this figure shows the nickel mesh It is completely covered by nic...
Embodiment 2
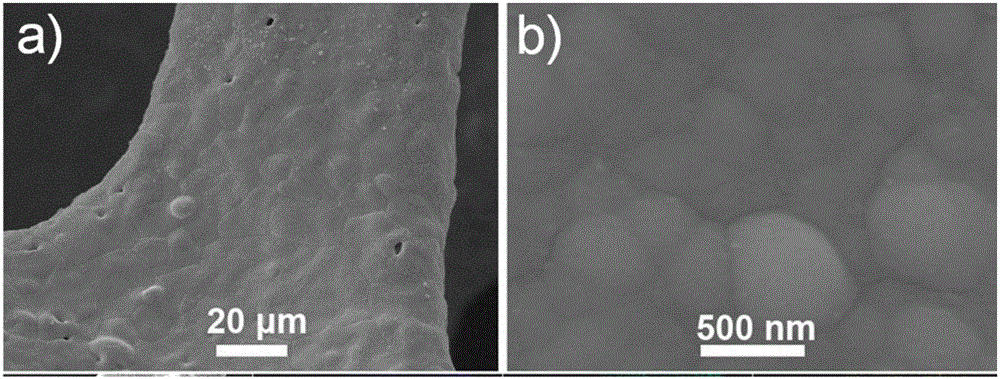
[0035] Weigh 0.05 moles of nickel nitrate, 0.2 moles of sodium citrate, 0.2 moles of sodium hypophosphite and 0.5 moles of ammonium sulfate and mix to obtain an electrolyte; wash the copper grid with dilute hydrochloric acid, ethanol, and deionization, and then add it to the above mixed solution , with copper mesh as the working electrode, graphite sheet as the counter electrode, and saturated calomel as the reference electrode, use an electrochemical workstation (CHI660D) at 25 ° C to 0.01 A / cm 2 The constant current was reacted for 1 hour, and nickel-phosphorus alloy particles were obtained on the copper grid.
[0036] image 3The scanning electron micrograph figure of the nickel-phosphorus alloy particle that is prepared in embodiment 2, wherein figure a is the scanning electron microscope photograph under the 20 micron scale, and figure b is the scanning electron microscope photograph under the 500 nanometer scale, and this figure shows the copper network Fully covered by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com