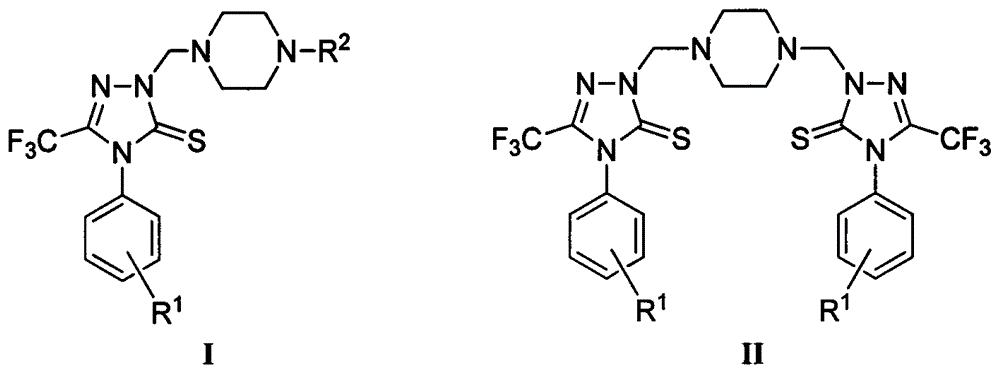
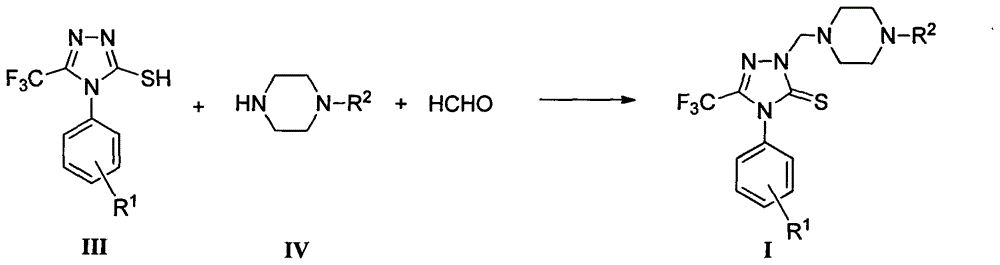
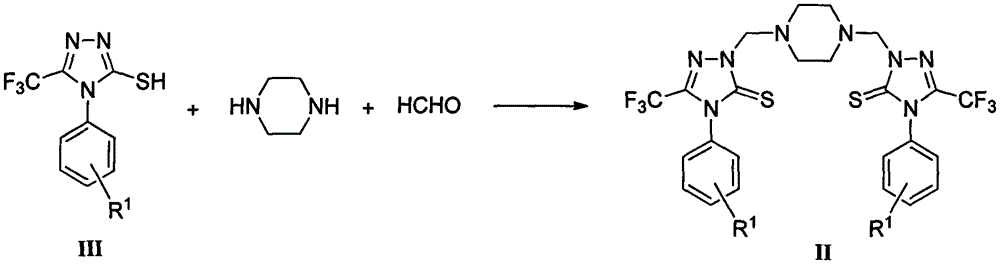
Triazole Mannich base compounds containing trifluoromethyl and piperazine, as well as preparation methods and application of triazole Mannich base compounds
A technology of trifluoromethyl compound, applied in the field of triazole Mannich base compounds, can solve the problems of bactericidal, herbicidal, and KARI enzyme inhibitory activity that has not been disclosed, and achieves good in vitro inhibitory activity effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation method of compound 03.
[0026] Step A: Preparation of N-(p-tolyl)thiosemicarbazide
[0027]
[0028] Add 4.80 g (0.12 mol) NaOH, 7.60 g (0.10 mol) CS to 10.73 g (0.10 mol) p-toluidine in DMF (100 mL) solution 2 , stirred at 20-25° C. for 1 h, raised the temperature to 60-70° C., slowly added 18.75 g (0.30 mol) of hydrazine hydrate (80%) and continued stirring for 1 h. After cooling, an appropriate amount of ice water was added, and a precipitate precipitated out. Suction filtration and ethanol recrystallization gave 9.70 g of brown crystals, with a yield of 54%.
[0029] Step B: Preparation of 4-(p-tolyl)-5-trifluoromethyl-4H-1,2,4-triazole-3-thiol
[0030]
[0031] 5.00g (27.58mmol) of N-(p-tolyl)thiosemicarbazide and 6.29g (55.17mmol) of trifluoroacetic acid were heated and stirred under reflux for 8 hours, and the reaction solution was poured into an appropriate amount of ice water, and a precipitate was precipitated, filtered by suction, and was...
Embodiment 2
[0036] Preparation method of compound 13.
[0037] Step A: Preparation of N-(p-fluorophenyl)thiosemicarbazide
[0038]
[0039]Add 4.80 g (0.12 mol) NaOH, 7.60 g (0.10 mol) CS to 11.11 g (0.10 mol) of p-fluoroaniline in DMF (100 mL) 2 , stirred at 20-25° C. for 1 h, raised the temperature to 60-70° C., slowly added 18.75 g (0.30 mol) of hydrazine hydrate (80%) and continued stirring for 1 h. After cooling, an appropriate amount of ice water was added, and a precipitate precipitated out. Suction filtration and ethanol recrystallization gave 10.50 g of brown crystals, with a yield of 57%.
[0040] Step B: Preparation of 4-(p-fluorophenyl)-5-trifluoromethyl-4H-1,2,4-triazole-3-thiol
[0041]
[0042] 5.00g (24.79mmol) N-(p-fluorophenyl) thiosemicarbazide and 6.16g (49.58mmol) trifluoroacetic acid were heated, stirred and refluxed for 8 hours. 6.12 g of colorless crystals were separated by petroleum ether-ethyl acetate (15:1) column chromatography, and the yield was 94%. ...
Embodiment 3
[0055] Determination of Bactericidal Activity by Isolated Plate Method
[0056] Put the test bacteria into flakes and insert them into a petri dish containing 50 μg / mL liquid medicine, put them in a biochemical incubator at 25°C for dark cultivation, and investigate the antibacterial effect after 3 days. Each treatment was repeated 3 times. Those who only added sterile water but no medicine were used as the control. The results are shown in Table 3. (%)
[0057] Inhibition rate (%) = [(blank colony diameter - treated colony diameter) ÷ (blank colony diameter - 4)] × 100%
[0058] Table 3. The in vitro bactericidal activity of the compound (50 μg / mL, inhibition rate %)
[0059] No.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com