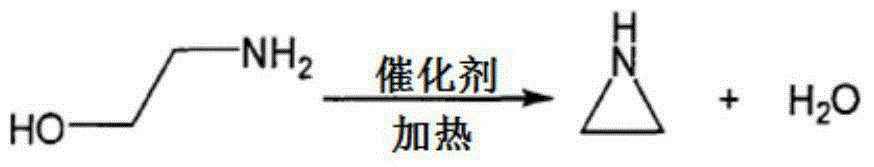
Monoethanolamine intramolecular dehydration catalyst
A technology of monoethanolamine and catalyst, which is applied in the field of catalysts for synthesizing aziridine by intramolecular dehydration of monoethanolamine. Improve diffusion effect, increase selectivity, reduce reaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Preparation of acid-activated montmorillonite
[0029] Disperse 16.33g montmorillonite in 800g mass fraction of 20% nitric acid aqueous solution, prepare a mass fraction of 2% montmorillonite suspension, stir, activate at 104°C for 24 hours, centrifuge at 8000 rpm for 6 Minutes, the precipitate was washed with distilled water until neutral, dried in an oven at 80°C for 12 hours, then baked in a muffle furnace at 900°C for 4 hours, cooled naturally to room temperature, and ground with a mortar to prepare acid-activated montmorillonite.
[0030] 2. Preparation of monoethanolamine intramolecular dehydration catalyst
[0031] Using equal volume impregnation method, according to Cs 3.0 P 2.4 La 1.0 o 18 The stoichiometric ratio of 0.26g cesium nitrate, 0.19g lanthanum nitrate hexahydrate, and 0.22g ammonium phosphate trihydrate were added to deionized water and allowed to stand for 15 minutes to obtain an impregnation solution; according to Cs 3.0 P 2.4 La 1.0 o 1...
Embodiment 2
[0033] 1. Preparation of acid-activated montmorillonite
[0034] Disperse 16.33g montmorillonite in 800g mass fraction of 20% nitric acid aqueous solution, prepare a mass fraction of 2% montmorillonite suspension, stir, activate at 104°C for 24 hours, centrifuge at 8000 rpm for 6 Minutes, the precipitate was washed with distilled water until neutral, dried in an oven at 80°C for 12 hours, then baked in a muffle furnace at 800°C for 4 hours, cooled naturally to room temperature, and ground with a mortar to prepare acid-activated montmorillonite.
[0035] 2. Preparation of monoethanolamine intramolecular dehydration catalyst
[0036] Using equal volume impregnation method, according to Cs 3.0 P 2.4 Ce 1.0 o 18 The stoichiometric ratio, 0.35g cesium nitrate, 0.26g cerium nitrate hexahydrate, 0.13g pyrophosphoric acid were added to deionized water, and left to stand for 15 minutes to obtain an impregnation solution; according to Cs 3.0 P 2.4 Ce 1.0 o 18 The loading capacit...
Embodiment 3
[0038] 1. Preparation of acid-activated montmorillonite
[0039] Disperse 20g of montmorillonite in 480g of 20% nitric acid aqueous solution by mass fraction to prepare a 4% montmorillonite suspension, stir, activate at 104°C for 24 hours, and centrifuge for 6 minutes at 8000 rpm , the precipitate was washed with distilled water until neutral, dried in an oven at 80°C for 12 hours, then baked in a muffle furnace at 650°C for 4 hours, cooled naturally to room temperature, and ground with a mortar to prepare acid-activated montmorillonite.
[0040] 2. Preparation of monoethanolamine intramolecular dehydration catalyst
[0041] Using equal volume impregnation method, according to Cs 3.0 P 2.4 PR 1.5 o 19.5 The stoichiometric ratio of 0.25g cesium nitrate, 0.38g praseodymium nitrate hexahydrate, and 0.14g diammonium hydrogen phosphate were added to deionized water, and left to stand for 15 minutes to obtain an impregnation solution; according to Cs 3.0 P 2.4 PR 1.5 o 19.5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com