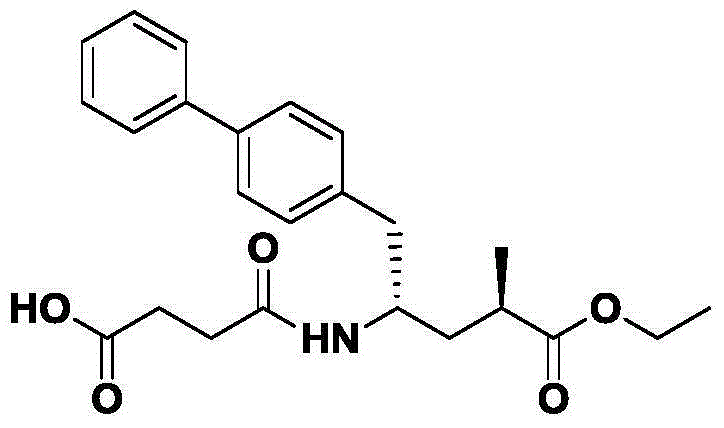
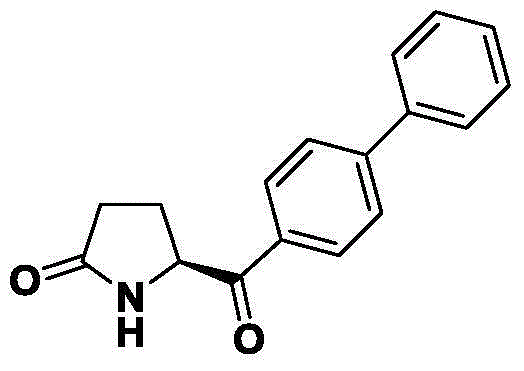
Synthetic method of Entresto midbody (S)-5-(diphenyl-4-carbonyl) pyrrolidine-2-ketone
A technology for pyrrolidine and intermediates, which is applied in the field of preparation of raw materials and intermediates, can solve the problems of increased cost, increased risk factor, large amount of consumption, etc., and achieves the effects of reducing production cost, shortening reaction period and improving production efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
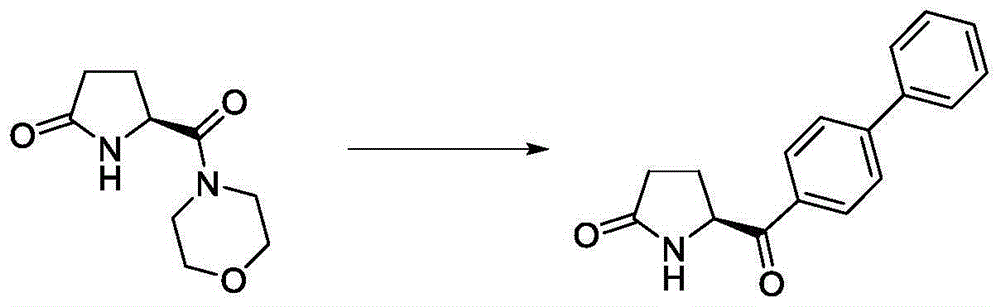
[0037] (1) Add 19.8g (0.1mol) of (S)-5-(morpholine-4-carbonyl)pyrrolidin-2-one into a 250mL three-necked flask, add solvent toluene (100mL), stir, and add triethylamine 10.6 g (0.105mol), turn on the heating, add 12.6g (0.1mol) of benzyl chloride dropwise at 50-60°C, raise the temperature to 80°C for 4 hours, and detect (S)-5-(morpholine-4-carbonyl) by HPLC ) pyrrolidin-2-one is less than 2%.
[0038] (2) Cool the reaction solution obtained in (1) to -15~-20°C, at this temperature, add 30.71 g (0.12 mol) of a tetrahydrofuran solution of biphenylmagnesium bromide dropwise, and keep the reaction for 5 hours after the addition is completed. Then add aluminum trichloride 20.0g (0.15mol) in batches under this condition, react for 1 hour, then slowly drop the reaction solution into the aqueous solution of 2N HCl to quench the reaction solution, static layering, ethyl acetate extracts the aqueous phase, The organic phases were combined, washed with saturated brine, dried, and concen...
Embodiment 2
[0040] (1) Add 19.8g (0.1mol) of (S)-5-(morpholine-4-carbonyl)pyrrolidin-2-one into a 250mL three-necked flask, add solvent toluene (100mL), stir, and add triethylamine 10.6 g (0.105mol), turn on the heating, add 16.9g (0.1mol) of benzyl bromide dropwise at 50-60°C, raise the temperature to 80°C for 4 hours, and detect (S)-5-(morpholine-4 -carbonyl)pyrrolidin-2-one is less than 2%.
[0041] (2) Cool the reaction solution obtained in (1) to -15~-20°C, at this temperature, add 30.71 g (0.12 mol) of a tetrahydrofuran solution of biphenylmagnesium bromide dropwise, and keep the reaction for 5 hours after the addition is completed. Then add aluminum trichloride 20.0g (0.15mol) in batches under this condition, react for 1 hour, then slowly drop the reaction solution into the aqueous solution of 2N HCl to quench the reaction solution, static layering, ethyl acetate extracts the aqueous phase, The organic phases were combined, washed with saturated brine, dried, and concentrated to d...
Embodiment 3
[0043](1) (S)-5-(morpholine-4-carbonyl)pyrrolidin-2-one 19.8g (0.1mol) was added to a 250mL three-necked flask, toluene (100mL) was added, stirred, and triethylamine 10.6 g (0.105mol), turn on the heating, add 12.6g (0.1mol) of benzyl chloride dropwise at 50-60°C, raise the temperature to 80°C for 4 hours, and detect (S)-5-(morpholine-4 -carbonyl)pyrrolidin-2-one is less than 2%,
[0044] (2) Cool the reaction solution obtained in (1) to -15~-20°C, at this temperature, add 30.71 g (0.12 mol) of a tetrahydrofuran solution of biphenylmagnesium bromide dropwise, and keep the reaction for 5 hours after the addition is completed. Then add palladium carbon 0.2g under this condition, feed hydrogen, and react at room temperature for 2 hours. After the reaction is completed, the palladium carbon will be filtered off and concentrated to dryness in vacuo to obtain 19.55 g of the product, yield: 73.6%, enantiomeric excess (ee) 98.15%, determined by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com