A silicon-based rhodamine nitric oxide fluorescent probe and its preparation method and application
A silicon-based rhodamine and nitric oxide technology, applied in the fields of chemistry and biology, can solve the problems of lack of lysosome-specific positioning function, low concentration, poor chemical stability, etc., achieve small damage, reduce interference, and penetrate powerful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
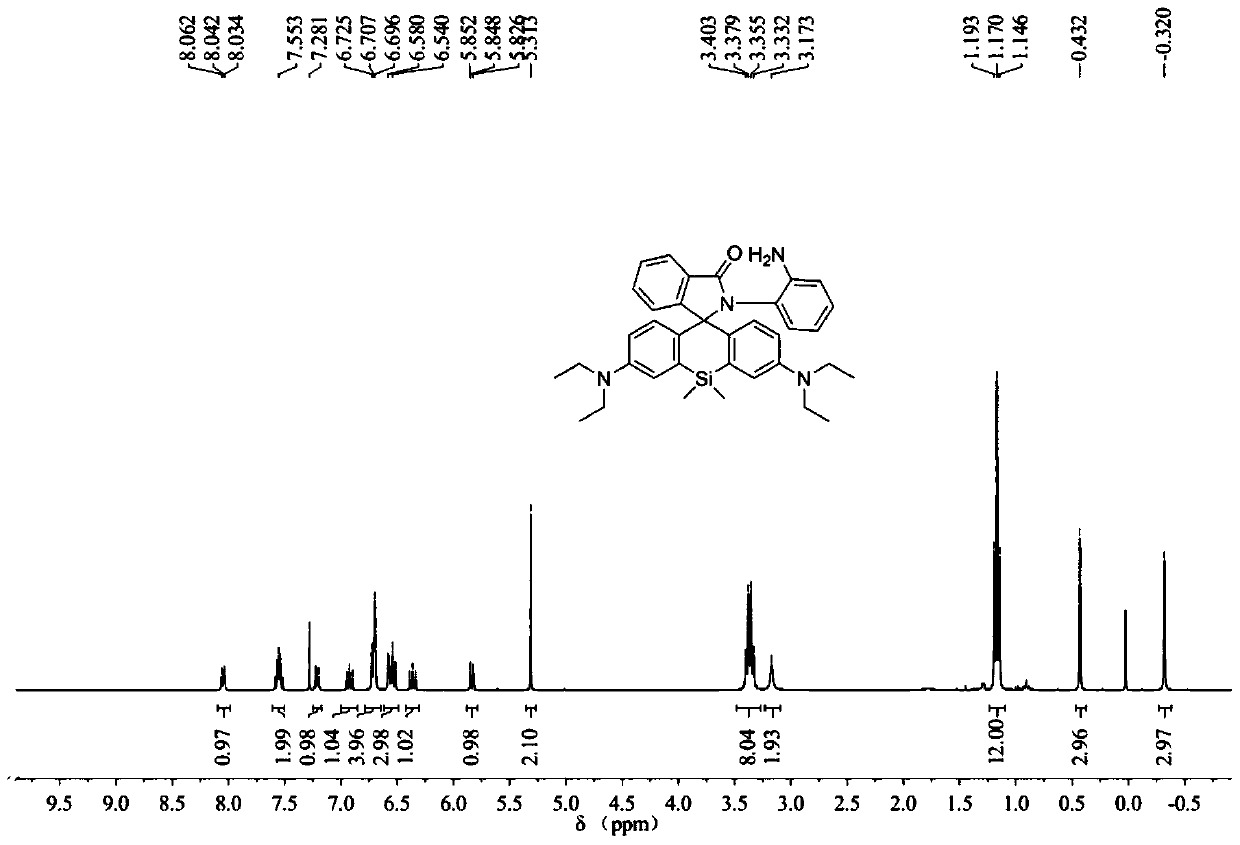
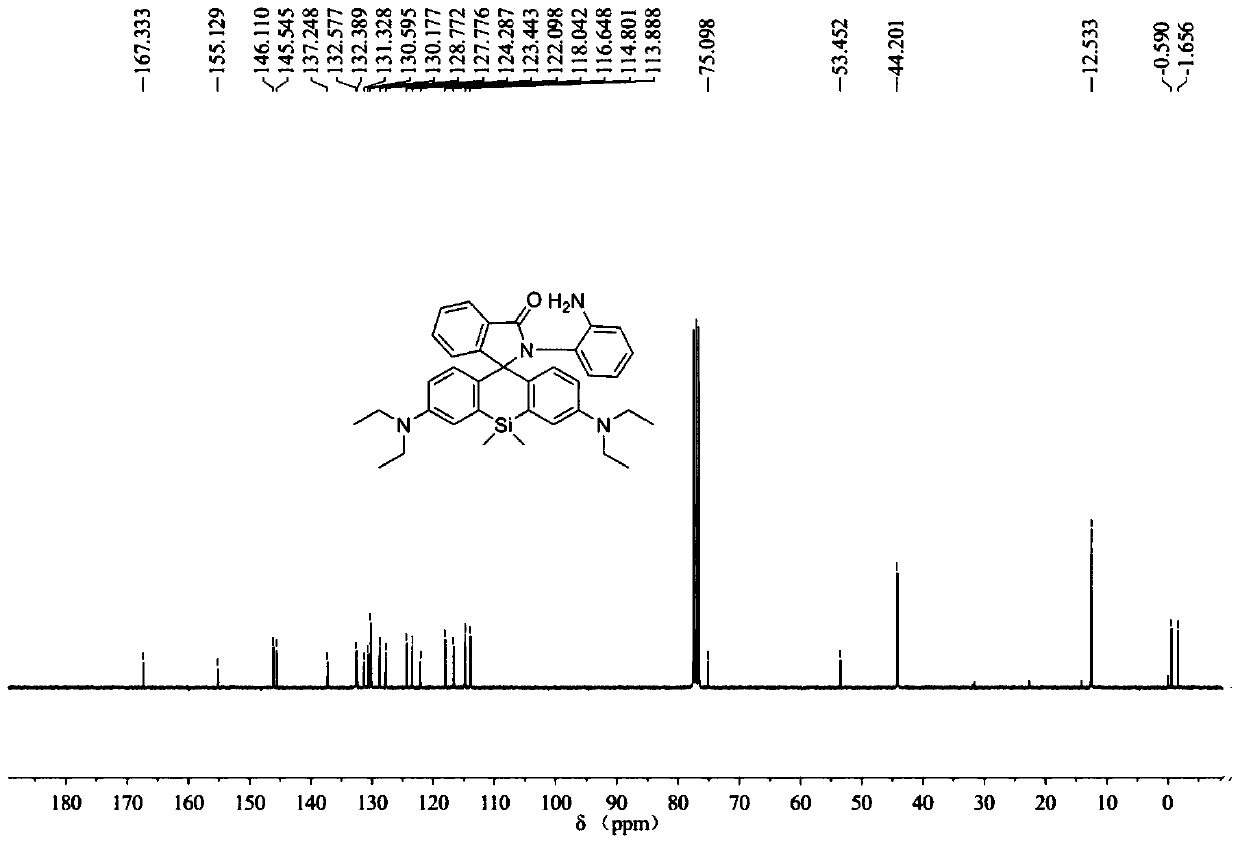
[0060] The synthesis of embodiment 1 probe SiRB-NO
[0061] In a 50ml reaction flask equipped with a magnetic stirrer, add raw material SiRB 727mg (1.5mmol), ClCH 2 CH 2 Cl (10ml) dissolved, argon protection, magnetic stirring, at room temperature, slowly add POCl 3 0.55ml, after the dropwise addition, heat to reflux, after 4 hours of reaction, stop heating, naturally cool to room temperature, evaporate the solvent under reduced pressure, add 10mL of acetonitrile to dissolve the remaining solid, and set aside. In another 50mL reaction flask, add 973mg (9.0mmol) of o-phenylenediamine, 5mL of acetonitrile, 5mL of triethylamine, magnetically stir, and under the protection of argon, slowly introduce the acetonitrile solution of the previously obtained solid into the reaction solution, React overnight at room temperature. The solvent was evaporated to dryness under reduced pressure, and the residue was washed with 30 mL CH 2 Cl 2 Dissolve, then add 30mL water, extract, separa...
Embodiment 2
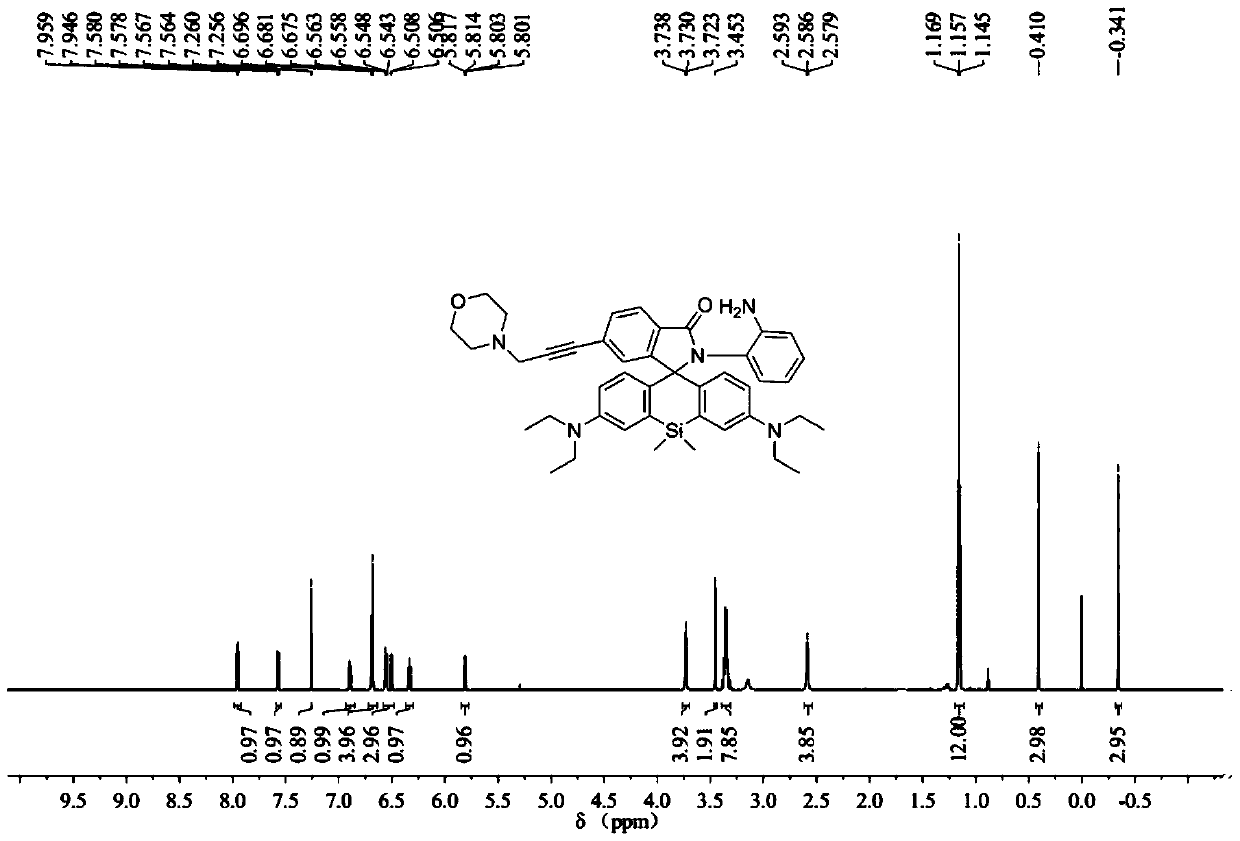
[0062] The synthesis of embodiment 2 probe Lyso-SiRB-NO
[0063] 1. Synthesis of Br-SiRB-NO:
[0064] In a 50mL reaction flask equipped with a magnetic stirrer, add 450mg (0.8mmol) of raw material Br-SiRB, ClCH 2 CH 2 Cl (5mL) dissolved, argon protection, magnetic stirring, at room temperature, slowly add POCl 3 0.3mL, after the dropwise addition, heat to reflux, after 4 hours of reaction, stop heating, naturally cool to room temperature, evaporate the solvent under reduced pressure, add 5mL of acetonitrile to dissolve the remaining solid, and set aside. In another 50mL reaction flask, add 519mg (4.8mmol) of o-phenylenediamine, 4mL of acetonitrile, 4mL of triethylamine, magnetically stir, and under the protection of argon, slowly introduce the acetonitrile solution of the previously obtained solid into the reaction solution, React overnight at room temperature. The solvent was evaporated to dryness under reduced pressure, and the residue was washed with 30 mL CH 2 Cl 2 ...
Embodiment 3
[0067] Example 3: Determination of Fluorescence Changes of Probe SiRB-NO at Different pH
[0068] 1. Probe SiRB-NO high standard solution configuration
[0069] Accurately weigh a certain amount of probe SiRB-NO, and use acetonitrile solvent to prepare a high-standard solution with a concentration of 0.5mM.
[0070] 2. Measurement of the fluorescence change of the probe SiRB-NO itself at different pH
[0071] Pipette 20 μL of the probe master solution and add 2000 μL of phosphate buffer (0.1M, containing 20% acetonitrile ), the fluorescence value was measured after 15 minutes, the excitation wavelength was 630nm, and the detection wavelength was 682nm. Fluorescent effect see attached Figure 5 .
[0072] 3. Determination of the effect of different pH on the recognition performance of SiRB-NO in nitric oxide
[0073] Pipette 20 μL of the probe mother solution into 2000 μL of phosphate buffer solution with different pH (2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 7.4, 8.0, 9.0, 10.0, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com