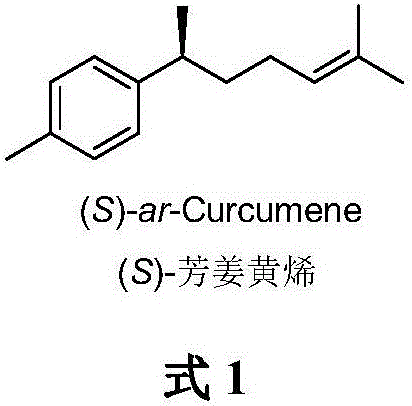
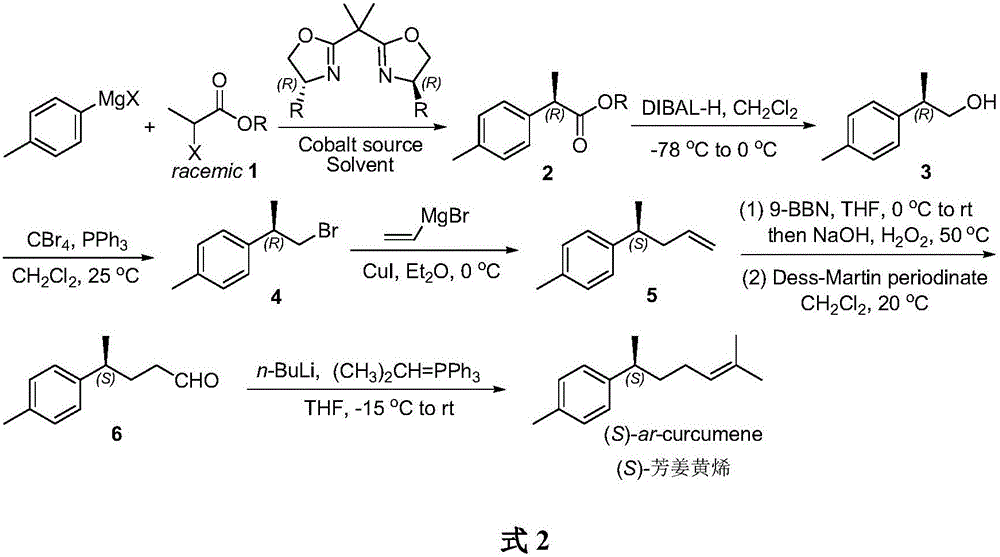
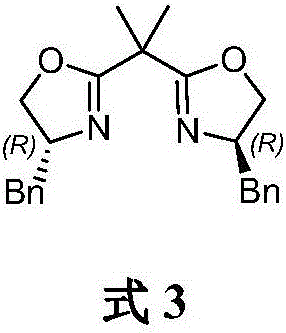
A method for asymmetric catalytic synthesis of (s) aromatic curcumene
An aromatic curcumene, asymmetric technology, applied in the field of new asymmetric catalytic synthesis - aromatic curcumene, can solve the problems of complicated reaction steps and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of (R)-benzyl p-toluene propionate 2
[0027] Under argon protection, add CoI to a 200mL Schlenk reaction flask 2 (0.63g, 2mmol), dried under vacuum for 30min. Add (4R,4'R)-bis(4-benzyloxazolin-2-yl)-propane L1 (0.87g, 2.4mmol) and anhydrous tetrahydrofuran (15mL), and stir at room temperature for 1h. Add racemic benzyl 2-bromophenylpropionate (4.86g, 20mmol) using a syringe, the temperature of the mixture drops to -78°C, slowly drop in p-tolylmagnesium bromide (30mL, 1.0M THF solution ,30mmol). Stirring was continued at -78°C for 8 h, and the reaction was quenched by adding saturated aqueous ammonium chloride (20 mL), and the organic phase was separated. The aqueous phase was extracted with diethyl ether (3 x 20 mL). The combined organic phases were washed with saturated NaCl solution. Anhydrous Na 2 SO 4Drying and concentration under reduced pressure gave the crude product, which was finally purified by silica gel column chromatography (petroleum ethe...
Embodiment 2
[0031] Synthesis of (R)-p-tolylpropanol 3
[0032] Under argon protection, add anhydrous CH to a 100 mL Schlenk reaction flask 2 Cl 2 (25 mL), (R)-benzyl p-tolylpropionate 2 (3.81 g, 15 mmol) was added by syringe. The mixture was lowered to -78°C, and diisobutylaluminum hydride (DIBAL-H) (22 mL, 1.5M solution in toluene, 33 mmol) was added dropwise using a syringe pump. After the dropwise addition was completed, the reaction was stirred at -78°C for 30 minutes, then the temperature was raised to 0°C, and the reaction was stirred for another 30 minutes. After the reaction, the temperature of the reaction solution was lowered to -78 °C, and the reaction was quenched with 1 mL of methanol. An aqueous potassium sodium tartrate solution (66 mL, 0.5 M, 33 mmol) was added, the temperature of the mixture was raised to room temperature and stirring was continued for 12 h. Separate the organic phase and use CH for the aqueous phase 2 Cl 2 (3 x 50 mL) extraction. The combined or...
Embodiment 3
[0034] Synthesis of (R)-2-p-tolyl-1-bromopropane 4
[0035] Add (R)-p-tolylpropanol 3 (1.50g, 10mmol), triphenylphosphine (3.15g, 12mmol) and CH to a 100mL reaction flask 2 Cl 2 (33 mL). Add CBr in batches with stirring 4 (3.42g, 10.3mmol), then the temperature of the reaction solution was raised to room temperature, and the stirring reaction was continued for 4h. After concentrating under reduced pressure, petroleum ether (40 mL) was added, suction filtered, the solid part was washed with a small amount of petroleum ether, and the filtrates were combined. with anhydrous Na 2 SO 4 Drying and concentration under reduced pressure gave the crude product, which was finally purified by silica gel column chromatography (petroleum ether) to obtain (R)-2-p-tolyl-1-bromopropane 4 (2.00g, yield 94%, optical 90% purity). [α] D 20 =19.3(c 1.8, CHCl 3 ). 1 H NMR (300MHz, CDCl 3 )δ7.14(d, J=8.3Hz, 2H), 7.10(d, J=8.3Hz, 2H), 3.56(dd, J=9.8, 6.1Hz, 1H), 3.46(dd, J=9.8, 8.0 Hz,1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com