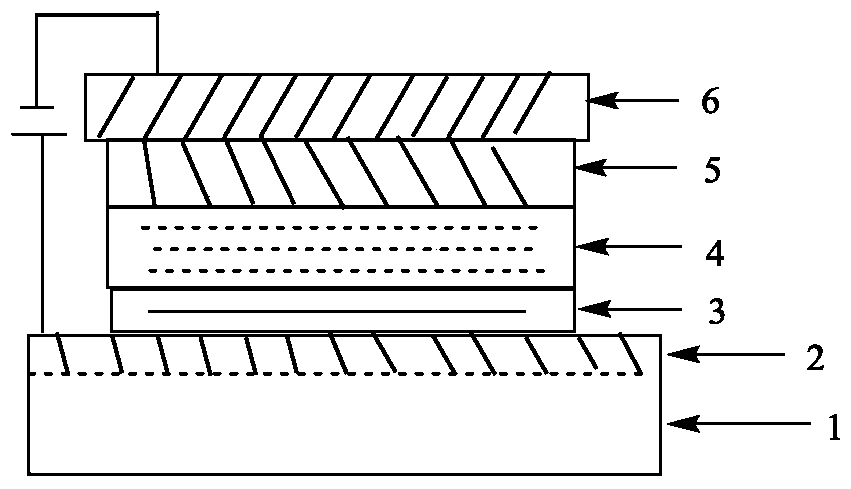
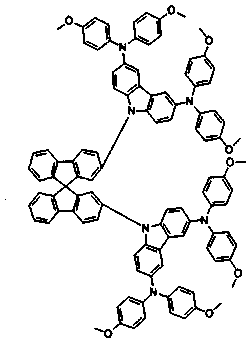
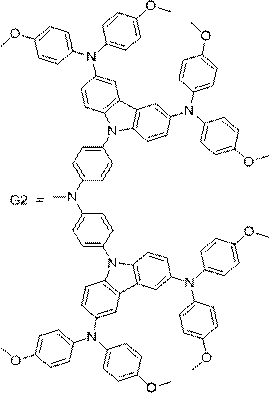
A kind of carbazole diarylamine dendritic compound and its preparation method and application
A carbazole diarylamine and compound technology, which is applied in the field of carbazole diarylamine dendrimers and their preparation, can solve the problems of deformation, easy decomposition of materials, low glass transition temperature, etc., and achieves improved hole transport. performance, good effect, effect of improving amorphous properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Preparation of Compound 1
[0055] The reaction route is as follows:
[0056]
[0057] Respectively through the following three schemes:
[0058] Solution 1: Add 23g (0.07mol) 3,6-dibromocarbazole and 23g (0.10mol) (BOC) to a 250mL three-necked flask 2 O, mix well to obtain mixture I, add 90g THF to the above mixture I, protect with nitrogen, stir mechanically, the system is completely dissolved, and the system is brown and clear at this time. 1.7g (0.014mol) DMAP was added to the system, after 5 minutes of adding, a lot of bubbles were generated, and the system became brown and clear. At this time, the oil bath was heated to 70°C, and the system was refluxed for 2h. After the reaction was completed, the temperature was reduced to 20-25°C, and the solvent was removed under reduced pressure to obtain 30.6 g of brown oil. It was dissolved in dichloromethane, filtered through a silica gel column, and rinsed with dichloromethane to obtain 28.9 g of white solid with a y...
Embodiment 2
[0061] Example 2 Preparation of Compound 2
[0062] The reaction route is as follows:
[0063]
[0064] Respectively through the following three schemes:
[0065] Scheme 1: Add 25.0g (0.058mol) of compound 1 (prepared by Scheme 1 of Example 1), 28.3g (0.12mol) 4,4'-dimethoxydiphenylamine, 17.0g into a 500mL three-necked flask (0.17mol) Sodium tert-butoxide, mix well to obtain mixture II, add 250g of toluene to mixture II, pass nitrogen protection, turn on mechanical stirring, stir evenly, the system is brown solution, add 0.53g (2.35×10 -3 mol) Palladium acetate and 0.95g (4.70×10 -3 mol) tri-tert-butyl phosphine, heated to 65°C, and reacted for 4 hours. After the reaction is completed, add 100ml of water to quench the reaction, stir for 15min, stand still for layering, extract the lower aqueous phase with 50g of toluene, combine the organic phases, and wash twice with water to pH=7, each time with 100ml of water, 15g of anhydrous Na 2 SO 4 Dry, filter on a silica gel column, rinse ...
Embodiment 3
[0068] Example 3 Preparation of Compound 3
[0069] The reaction route is as follows:
[0070]
[0071] Respectively through the following three schemes:
[0072] Scheme 1: Add 31.1g (0.043mol) of compound 2 (prepared by Scheme 1 of Example 2) and 14.5g (0.129mol) of potassium tert-butoxide into a 1L three-necked flask, and mix well to obtain mixture Ⅲ. Add 620g of toluene to Ⅲ, turn on stirring, heat to 100°C, after 1 hour of reaction, the reaction is completed, down to 20-25°C, add 300g of water to quench the reaction, separate layers, 200g of ethyl acetate to extract the aqueous phase, and combine the organic phases , Dry with anhydrous sodium sulfate, remove the solvent under reduced pressure to obtain 31.3g dark green solid, 260g of methylene chloride dissolve the dark green solid, pass through a silica gel column, dichloromethane elution, remove the solvent under reduced pressure to obtain 28.3g yellow solid, toluene Recrystallized with a mixed solvent of n-hexane (mass ratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com