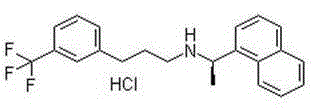
Cinacalcet hydrochloride containing pharmaceutical composition and preparation method thereof
A technology of cinacalcet hydrochloride and its composition, which is applied in the field of pharmaceutical composition containing cinacalcet hydrochloride and its preparation, to achieve the effects of good stability, increased drug safety, and accelerated disintegration rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
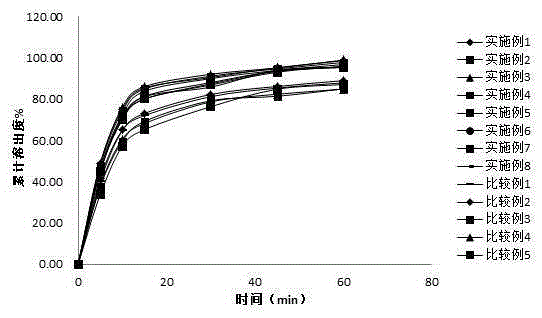
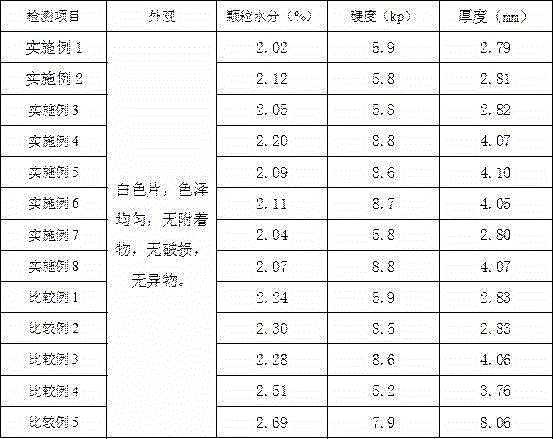
Image
Examples
Embodiment 1
[0030] Preparation prescription: 25mg dose
[0031] Element Dosage (g) proportion% Cinacalcet hydrochloride 27.5 34.4% pregelatinized starch 21.5 26.9% microcrystalline cellulose 21.5 26.9% Crospovidone-internal 3.125 3.9% Crospovidone - Extra 1.875 2.3% purified water 30.0 -- Methylcellulose 3.0 3.7% Polysorbate 0.5 0.6% Magnesium stearate 1.0 1.3% Chip 80 100%
[0032] Tablet core preparation: Weigh cinacalcet hydrochloride, microcrystalline cellulose, pregelatinized starch, and cross-linked povidone according to the prescription, mix evenly, add 30g of purified water as a soft material for binder, and use a 20-mesh sieve Granulate, dry in an oven at 55-65°C, sieve through a 24-mesh sieve for granulation, add methylcellulose, polysorbate, and magnesium stearate, mix evenly, and compress into tablets to obtain tablet cores.
[0033] Coating: Prepare a coating solution with a concentration ...
Embodiment 2
[0035] Preparation prescription: 25mg dose
[0036] Element Dosage (g) proportion% Cinacalcet hydrochloride 27.5 34.4% pregelatinized starch 30.0 37.5% microcrystalline cellulose 15.0 18.7% Crospovidone-internal 1.5 1.9% Crospovidone - Extra 1.5 1.9% purified water 30.0 -- Methylcellulose 0.5 0.6% Polysorbate 3.0 3.7% Magnesium stearate 1.0 1.3% Chip 80 100%
[0037] Tablet core preparation: Weigh cinacalcet hydrochloride, microcrystalline cellulose, pregelatinized starch, and cross-linked povidone according to the prescription, mix evenly, add 30g of purified water as a soft material for binder, and use a 20-mesh sieve Granulate, dry in an oven at 55-65°C, sieve through a 24-mesh sieve for granulation, add methylcellulose, polysorbate, and magnesium stearate, mix evenly, and compress into tablets to obtain tablet cores.
[0038] Coating: Prepare a coating solution with a concentration of 1...
Embodiment 3
[0040] Preparation prescription:
[0041] Element Dosage (g) proportion% Cinacalcet hydrochloride 27.5 34.4% pregelatinized starch 15.0 18.7% microcrystalline cellulose 30.0 37.5% Crospovidone-internal 2.0 2.5% Crospovidone - Extra 1.0 1.3% purified water 30.0 -- Methylcellulose 2.0 2.5% polyvinyl alcohol 2.0 2.5% Magnesium stearate 0.5 0.6% Chip 80 100%
[0042]Tablet core preparation: Weigh cinacalcet hydrochloride, microcrystalline cellulose, pregelatinized starch, and cross-linked povidone according to the prescription, mix evenly, add 30g of purified water as a soft material for binder, and use a 20-mesh sieve Granulate, dry in an oven at 55-65°C, sieve through a 24-mesh sieve for granulation, add methyl cellulose, polyvinyl alcohol, and magnesium stearate, mix evenly, and compress into tablets to obtain tablet cores.
[0043] Coating: Prepare a coating solution with a concentration of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com