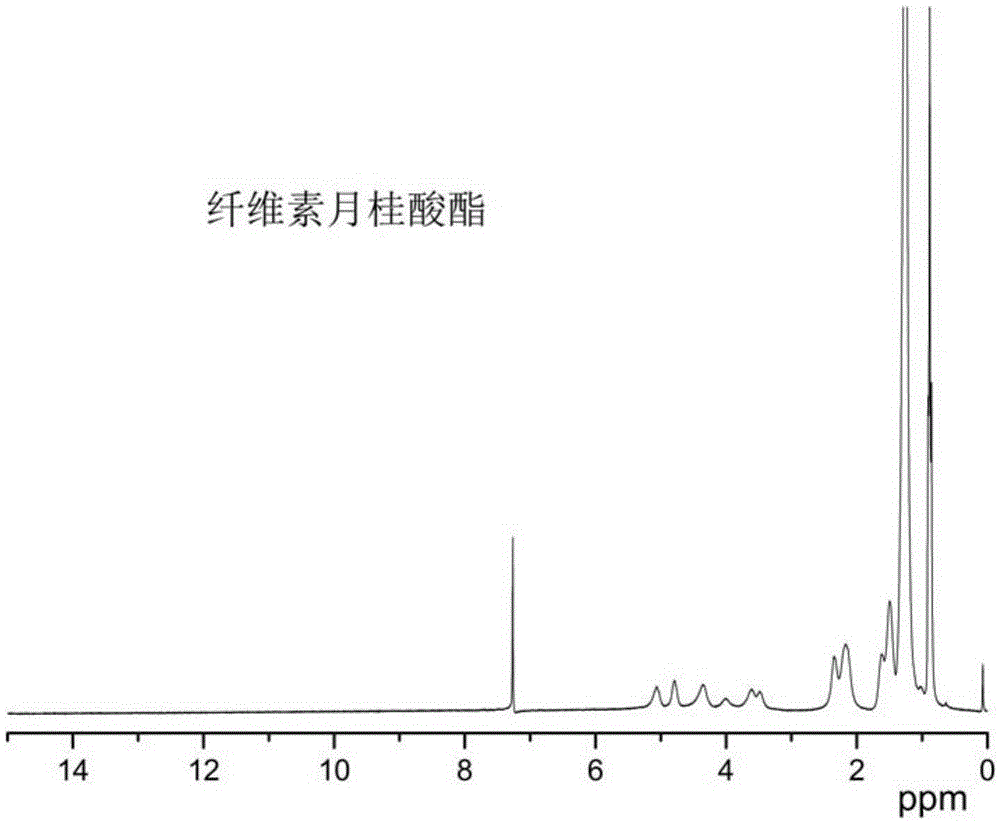
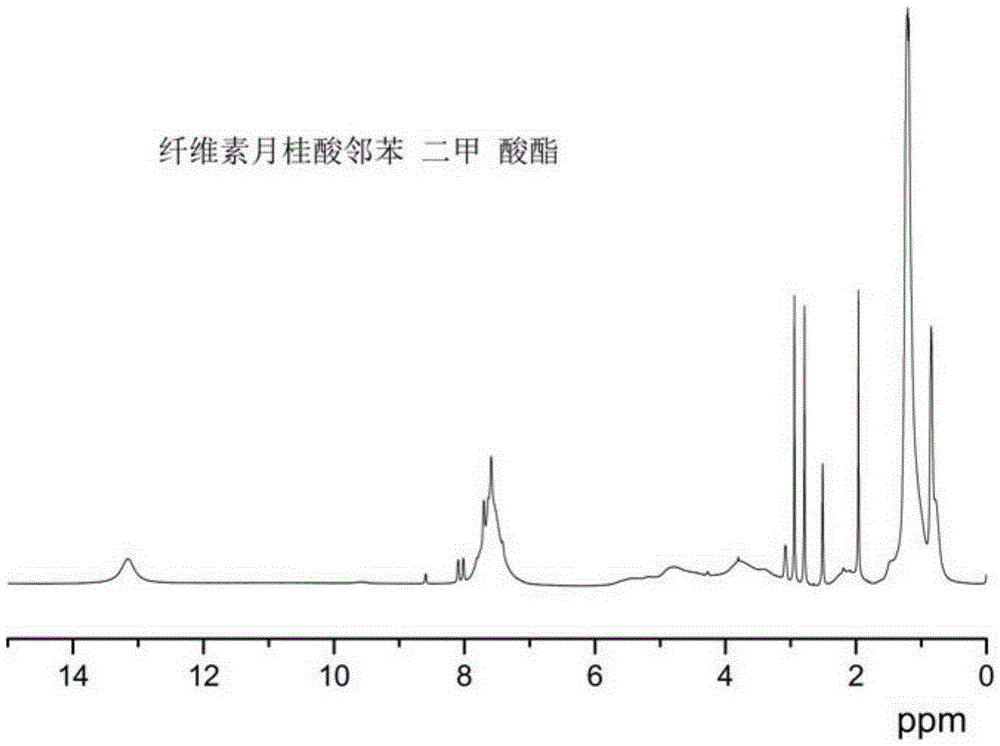
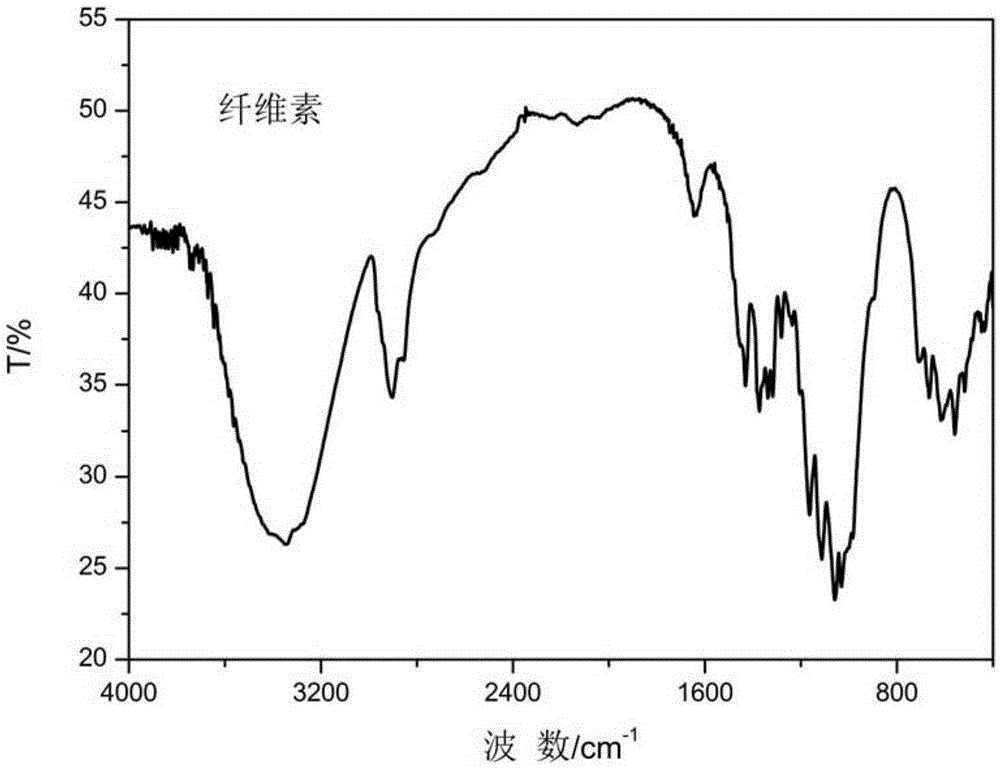
Cellulose long chain fatty acid phthalate and synthesis preparation method therefor
A technology of phthalates and long-chain fatty acids, which is applied in the field of new cellulose esters and their synthesis and preparation, can solve problems such as no synthetic process methods, avoid potential harm to the human body, overcome dependence on plasticizers, The effect of process condition moderation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Synthesis of cellulose caprylic acid phthalate with homogeneous cellulose DMAc / LiCl solution:
[0020] Step 1, the synthesis of cellulose octanoate:
[0021] Put 50 g of cellulose DMAc / LiCl solution with a mass concentration of 2% into a 100 ml three-necked flask, and start the stirring device; the molar ratio of cellulose anhydroglucose unit (AGU) to octanoyl chloride n(AGU):n(octanoyl chloride ) at a ratio of 1:2, add a certain amount of octanoyl chloride, and then add 5ml of acid-binding agent pyridine to reduce the degradation effect of hydrochloric acid generated by the reaction on cellulose, react at a temperature of 50°C for 12 hours, and then cool to room temperature for use ;
[0022] Step 2: Add 2 g of phthalic anhydride and 5 ml of catalyst triethylamine to the system after the reaction in Step 1, stir and react for 30 minutes at room temperature, and then use an aqueous solution with a concentration of 35% hydrochloric acid and water at a volume ratio of 1:...
Embodiment 2
[0025] Synthesis of cellulose nonanoic acid phthalate with homogeneous cellulose DMAc / LiCl solution;
[0026] Step 1: Synthetic reaction of cellulose nonanoate
[0027] Add 50 g of cellulose DMAc / LiCl solution with a mass concentration of 2% to a 100 ml three-necked bottle, and use the molar ratio of cellulose anhydroglucose unit: nonanoic acid: p-toluenesulfonyl chloride n(AGU):n(nonanoic acid): n (p-toluenesulfonyl chloride) is 1:4:4. Add nonanoic acid and p-toluenesulfonyl chloride. The p-toluenesulfonyl chloride is a solution dissolved in 5ml of DMAc. Add 6ml of pyridine to reduce and weaken the hydrochloric acid produced in the reaction. For the effect on the degradation of cellulose, stir and react at 50°C for 12 hours, then cool to room temperature and set aside;
[0028] Step 2: Add 2 g of phthalic anhydride and 5 ml of catalyst triethylamine to the system after the reaction in Step 1, stir and react for 30 minutes at room temperature, and then use an aqueous solution...
Embodiment 3
[0031] Synthesis of cellulose capric acid phthalate with homogeneous cellulose DMAc / LiCl solution:
[0032] Step 1: Synthesis of cellulose caprate:
[0033]Add 50 g of cellulose DMAc / LiCl solution with a mass concentration of 2% into a 100 ml three-necked flask, turn on the stirring device, and the molar ratio of the anhydroglucose unit of cellulose: capric acid: p-toluenesulfonyl chloride n(AGU):n( Decanoic acid): n (p-toluenesulfonyl chloride) is 1:4:4. Add capric acid and p-toluenesulfonyl chloride, wherein p-toluenesulfonyl chloride is a solution dissolved in 5ml of DMAc, and then add 6ml of acid-binding agent pyridine to reduce, To weaken the effect of hydrochloric acid produced in the reaction on the degradation of cellulose, raise the temperature to 50°C and maintain the reaction for 12 hours, then cool down to room temperature, pour it into distilled water to precipitate, filter with suction, and wash the filtered product with ethanol at 50°C for two to three times , ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Saponification value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com