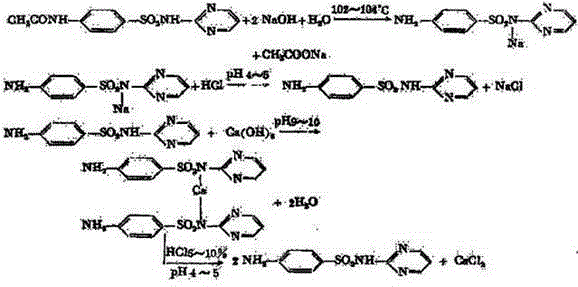
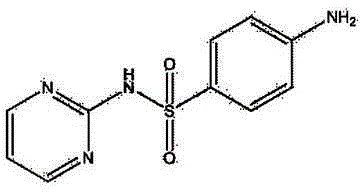
Synthetic method for sulfadiazine
A technique for the synthesis of sulfadiazine, which is applied in the field of organic chemical synthesis, can solve the problems of hindering the smooth progress of the condensation reaction, weakening the condensation efficiency of pyridine, and long condensation reaction time, etc., and achieves the effects of easy acquisition, high yield, and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: a kind of synthetic method of sulfadiazine comprises the following steps:
[0030] A will be 8.6gNH 4 Cl with 23.1g (NH 4 ) 2 CO 3 After mixing according to a certain ratio, add activated carbon catalyst, then add 32g of dicyandiamide and heat to melt, keep at 150°C for 30min;
[0031] B Add 80g of p-sulfamic acid to the above molten liquid, continue to melt 70g of sodium carbonate, raise the temperature to 150°C within 25min, and keep it for 30min;
[0032] C. Add 400ml of boiling water to the mixture obtained in step B. After stirring evenly, cool the obtained suspension to 40° C. and filter to obtain the crude sulfamidine.
[0033] Add 93g of 25% sodium methoxide methanol solution and 33g of crude sulfamidine to the flask in turn, add 13.5g of malondialdehyde under stirring, raise the temperature to 65°C, and react for 2h to complete the ring condensation reaction. After recovering methanol, the crude sulfadiazine is obtained. Add 200ml of water to...
Embodiment 2
[0034] Embodiment 2: a kind of synthetic method of sulfadiazine comprises the following steps:
[0035] A will be 8.6gNH 4 Cl with 30.9g (NH 4 ) 2 CO 3 After mixing according to a certain ratio, add activated carbon catalyst, then add 32g of dicyandiamide and heat to melt, and keep at 170°C for 30min;
[0036] B Add 80g of p-sulfamic acid to the above molten liquid, continue to melt 70g of sodium carbonate, raise the temperature to 150°C within 25min, and keep it for 30min;
[0037] C. Add 400ml of boiling water to the mixture obtained in step B. After stirring evenly, cool the obtained suspension to 40° C. and filter to obtain the crude sulfamidine.
[0038] Add 93g of 25% sodium methoxide methanol solution and 33g of crude sulfamidine to the flask in turn, add 12.0g of malondialdehyde under stirring, raise the temperature to 70°C, and react for 2.5h to complete the ring condensation reaction. After recovering methanol, crude sulfadiazine is obtained. Add 200ml of water ...
Embodiment 3
[0039] Embodiment 3: a kind of synthetic method of sulfadiazine comprises the following steps:
[0040] A will be 8.6gNH 4 Cl with 46.2g (NH 4 ) 2 CO 3 After mixing according to a certain ratio, add activated carbon catalyst, then add 32g of dicyandiamide and heat to melt, and keep at 180°C for 30min;
[0041]B Add 80g of p-sulfamic acid to the above molten liquid, continue to melt 70g of sodium carbonate, raise the temperature to 150°C within 25min, and keep it for 30min;
[0042] C. Add 400ml of boiling water to the mixture obtained in step B. After stirring evenly, cool the obtained suspension to 40° C. and filter to obtain the crude sulfamidine.
[0043] Add 100 g of 25% sodium methoxide methanol solution and 40 g of crude sulfadiazine to the flask in turn, add 14.5 g of malondialdehyde under stirring, raise the temperature to 60°C, and react for 4 hours to complete the ring condensation reaction. After recovering methanol, the crude sulfadiazine is obtained. Add 200m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com