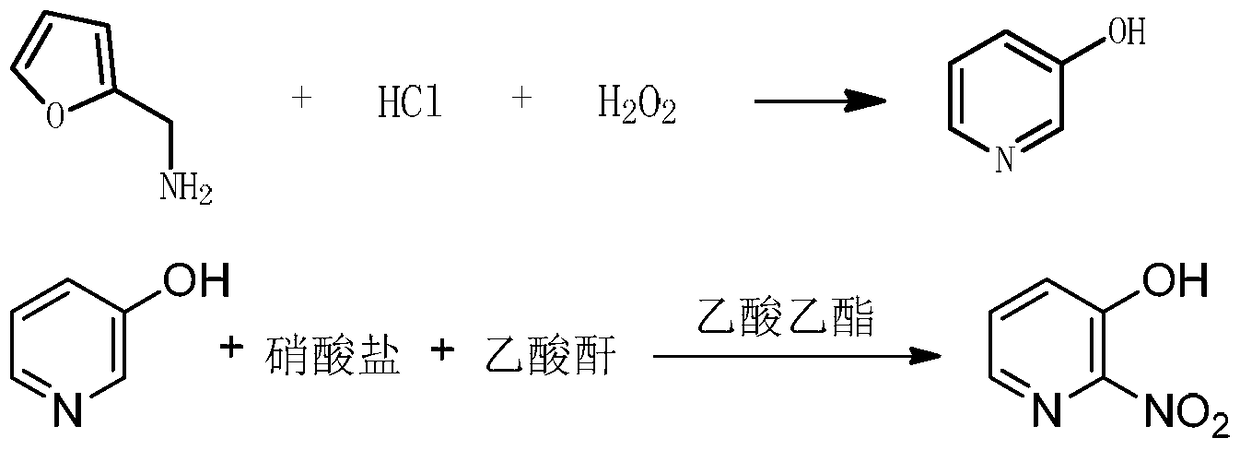
A kind of synthesis technique of 3-hydroxy-2-nitropyridine
A technology of nitropyridine and hydroxypyridine, applied in the field of synthesis technology of 3-hydroxy-2-nitropyridine, can solve the problems of low yield, excessive use of strong acid, etc., and achieves improved reaction yield, improved yield, The effect of shortening the post-processing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of 3-hydroxypyridine.
[0034] A thermometer, a reflux condenser, a constant pressure dropping funnel, and a magnetically stirred 250ml three-necked flask were added with 85ml of 20% hydrochloric acid solution (0.510mol), and then slowly added dropwise 10g of furfurylamine (0.102mol). After the addition is completed, cool to between 10°C and slowly add 19ml of 30% concentration of H 2 o 2 , the dropping time is 30-40 minutes. After the dropping, the holding time is 1.5 hours, heated to 100°C, heated to reflux for 2.5-3 hours, spotting the sample to monitor the reaction end point, cooling to room temperature after the reaction, and using 1mol / L NaOH The pH was adjusted to 7-8, and 8.1 g of 3-hydroxypyridine was obtained by repeated extraction with ether, with a yield of 83%.
[0035] Replacement example 1-1-1-9:
[0036] The preparation method is the same as in Example 1, except that the concentration of hydrochloric acid is adjusted, the temper...
Embodiment 2
[0040] Example 2: Preparation of 3-hydroxy-2-nitropyridine.
[0041] 10g of 3-hydroxypyridine (105mmol) and 80ml of ethyl acetate and 4.2g of KNO 3 (42mmol) and 21ml of acetic anhydride (0.210mol) were added to a 250mL three-neck flask, heated at a temperature of 45°C with magnetic stirring to react, and the sample was monitored to monitor the end of the reaction. Wash the ester 1-2 times, take the filtrate and adjust the pH to neutral with NaOH saturated solution, extract 3-4 times with ethyl acetate, take the extract and add activated carbon and heat it under reflux for 1 hour, cool and filter, take the filtrate with anhydrous magnesium sulfate Dry, filter, concentrate on a rotary evaporator, and dry in a drying oven. 11.9 g of 3-hydroxy-2-nitropyridine was obtained with a yield of 81%.
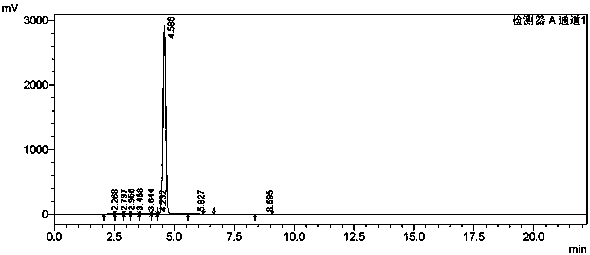
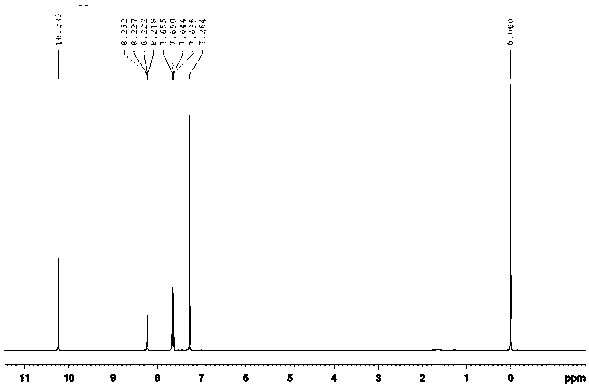
[0042]The nuclear magnetic resonance spectrum and high performance liquid chromatography of the product are as follows: figure 1 , figure 2 shown.
[0043] Replacement examples 2-1 to...
Embodiment 3
[0048] Example 3: Preparation of 3-hydroxy-2-nitropyridine.
[0049] 50g of 3-hydroxypyridine (0.525mol) and 400ml of ethyl acetate and 74g of KNO 3 (0.735mol) and 367ml of acetic anhydride (3.675mol) were added to a 1L three-neck flask, heated and stirred mechanically at a temperature of 45°C to react, and the sample was monitored to monitor the end of the reaction. Wash 1-2 times with ethyl ester, take the filtrate and adjust the pH to neutral with NaOH saturated solution, extract 3-4 times with ethyl acetate, take the extract and add activated carbon to reflux for 1 hour, cool and filter, take the filtrate with anhydrous sulfuric acid Magnesium was dried, filtered, concentrated on a rotary evaporator, and then dried in a drying oven. 66 g of 3-hydroxy-2-nitropyridine was obtained with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com