D-acetylglucosamine deacetylase heterologous expression and application
A deacetylase and glucosamine technology, applied in the direction of microbial-based methods, hydrolase, recombinant DNA technology, etc., to achieve the effect of large industrialization potential, strong substrate specificity, and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
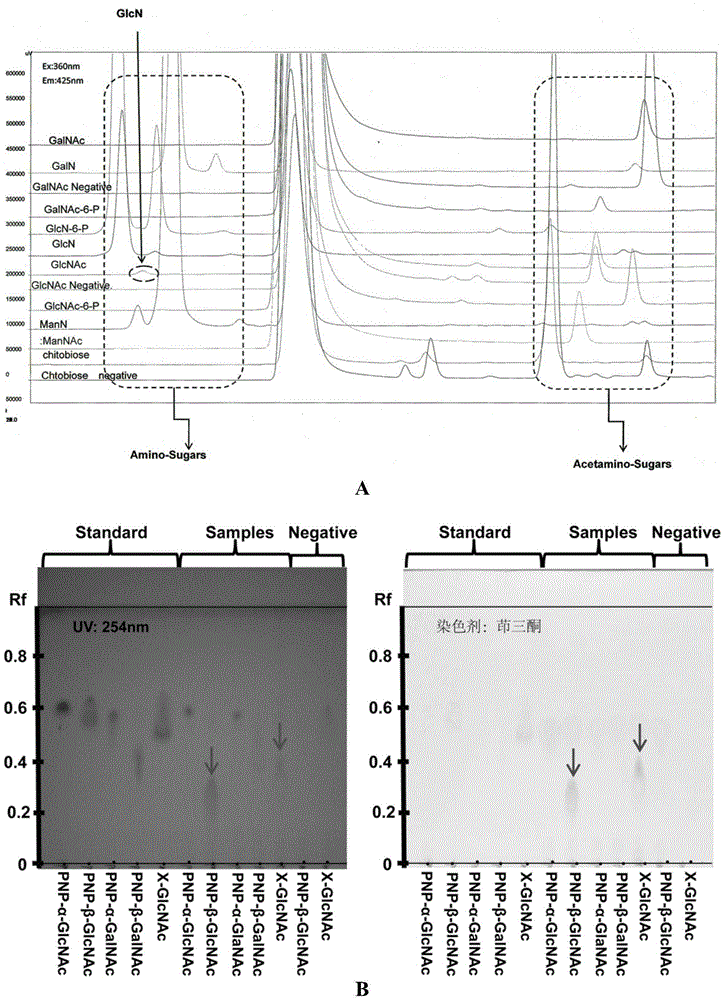
[0044] The acquisition of embodiment 1D-acetylglucosamine deacetylase gene:
[0045] (1) Dip a little Cyclobacterium marinum 1.5mL centrifuge tube with a pipette gun, add 500μl marine round bacteria DNA extraction solution (50mM Tris-base, 50mM sodium chloride, 500mM EDTA, 110% SDS, 1% Triton-X100 and 0.2mU / μl Proteinase K, pH8.0), inactivate proteinase K at 90°C after incubating at 55°C for 3 hours. Store at -20°C for later use.
[0046] (2) Obtain the target gene by PCR
[0047] Primers were designed according to the gene of D-acetylglucosamine deacetylase in GeneBank, with restriction endonuclease EcoRI and XhoI base sequences and protective bases at both ends of the primers. Synthesized by Nanjing GenScript Company:
[0048] EcoRIF: 5'CGGAATTCATGAATGCAGCACAAAAATTAG3'
[0049] XhoIR: 5'CCGCTCGAGTTAGTCTTTATATATTTTTTCCCT3'
[0050] The PCR program is: 95°C for 10 seconds, 55°C for 15 seconds, and 72°C for 1 minute, a total of 35 cycles. After the PCR reaction, run elect...
Embodiment 2
[0053] Expression of embodiment 2D-acetylglucosamine deacetylase coding gene in BL (DE21)
[0054] Extract the plasmid from the Escherichia coli Top10 cells carrying the recombinant plasmid obtained in Example 1, and transform it into the prepared competent cell BL21(DE3). Pick the recombinant Escherichia coli strain BL21 (DE3) into 5 ml of LB liquid medium containing kana antibiotics at 37° C. and shake at 250 rpm for overnight culture. Transfer to fresh LB (400ml) culture medium according to 1% inoculum size (v / v), culture at 37°C, shake at 250rpm until OD 600 ≈0.6-0.8, add the inducer IPTG to a final concentration of 1.0mM, culture at 25°C and 250rpm for 3 hours with shaking.
Embodiment 3
[0055] Example 3D-Purification of acetylglucosamine deacetylase
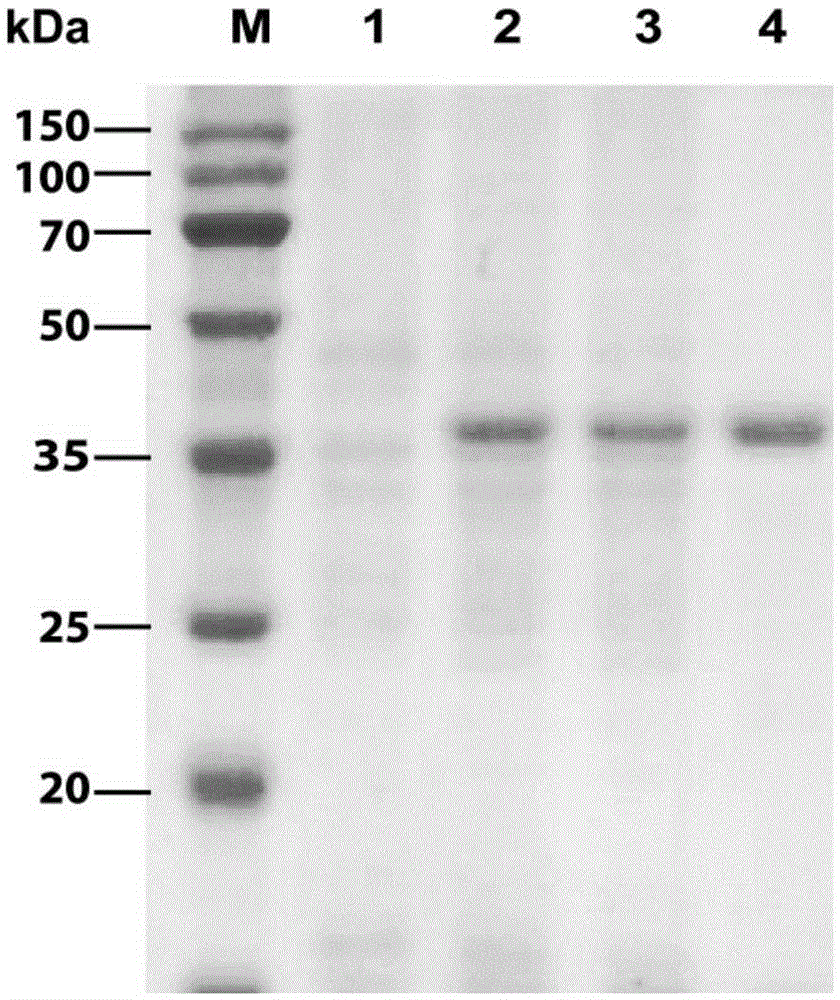
[0056] The bacterium solution of the D-acetylglucosamine deacetylase expressed in Example 2 was centrifuged at 4° C. at 4000 rpm for 20 min to collect the thalline, and 10 ml of lysis buffer (pH 7.550 mM sodium chloride; 50 mM Tris-HCl ; 1% Triton), 100 μl PMSF, resuspending the bacteria in the lysate, and breaking the cells in an ultrasonic breaker for 20 minutes. The broken cell lysate was centrifuged at 4000rpm for 20min at 4°C to collect the supernatant.
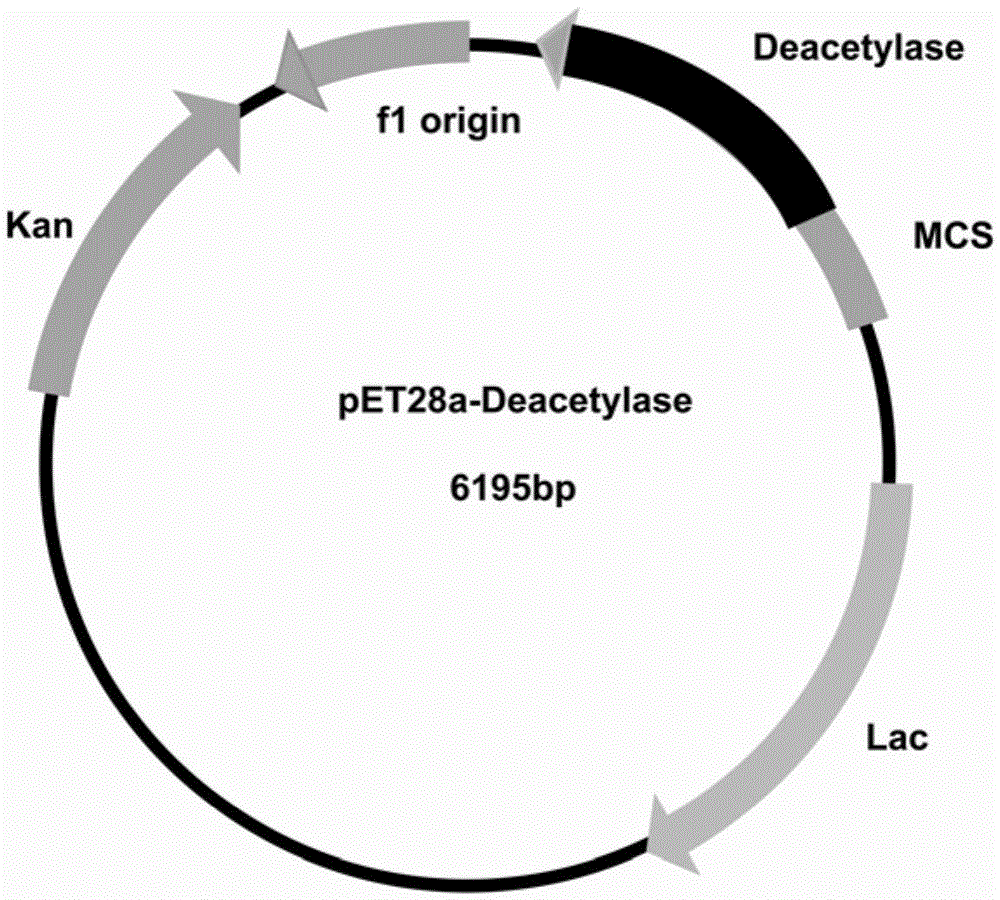
[0057] Since the N-terminal of the designed recombinant expression vector pET28a(+)-N-acetylglucosaminedeacetylase expression product has 6 consecutive histidines, it can be affinity-purified by an affinity chromatography column (Ni-NTA agarose gel). First equilibrate the column (pH8.0100mM sodium chloride 50mMTris-HCl) with an equilibrium solution, load the crude enzyme solution onto the column; use 10 times the volume of washing solution (pH8.0100mM sod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com