Chinese loropetalum extract comprising quinate and glucoside compounds and pharmaceutical application thereof
A technology of extract and quinic acid, which is used in the treatment of bleeding diseases and the preparation of hemostatic drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Implementation Example 1: Preparation of the total extract of Roropetus alba and different polar parts
[0039] Take 20kg of dried medicinal materials of P. chinensis, cut into 2-3cm segments, soak and extract 3 times with 8-10 times acetone at room temperature, 5 days each time, combine the extracts, concentrate under reduced pressure to dryness, and obtain 902g of total extract of P. . Weigh 793.5 g of acetone extract, add 4 L of deionized water for ultrasonic dispersion, and add 4 L of petroleum ether for extraction 5 times. The water layer was evaporated to dryness to obtain 564.5 g of the water-soluble fraction. Add 2L of deionized water to dissolve, apply HP20 macroporous resin after filtration, the ratio of resin column diameter to height is 1:8, use 10%, 20%, 30%, 40%, 50%, 70% of 2 times BV after sample loading 95% ethanol-water gradient elution, collect 20%, 30%, 40% ethanol eluate and evaporate to dryness under reduced pressure to obtain 20%, 30%, 40% ethan...
Embodiment 2
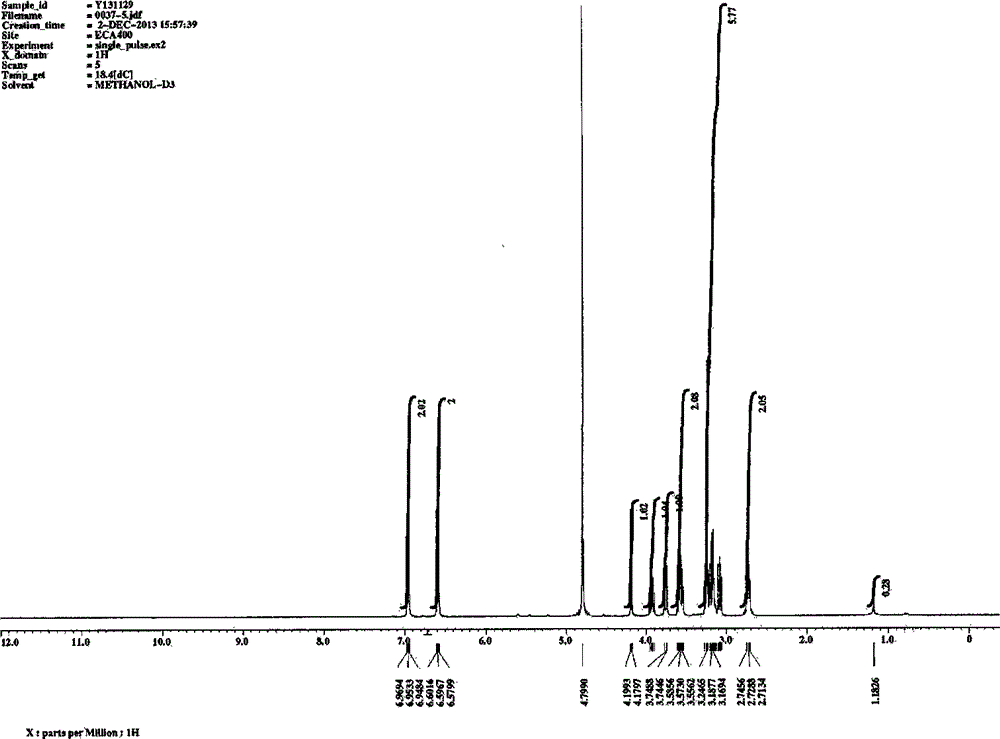
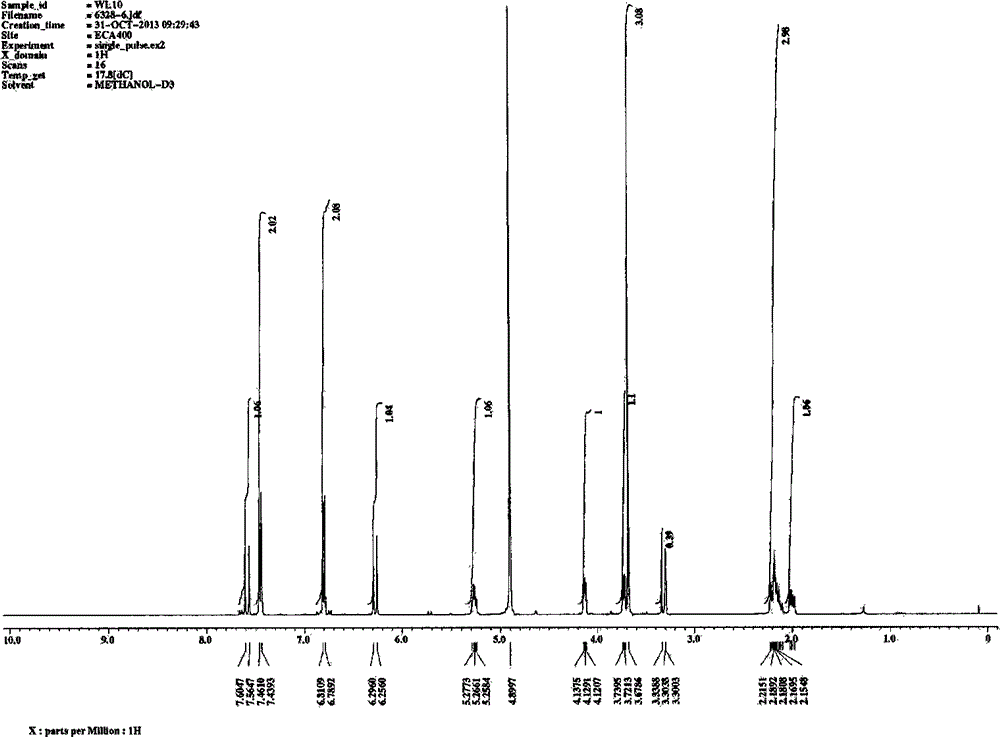
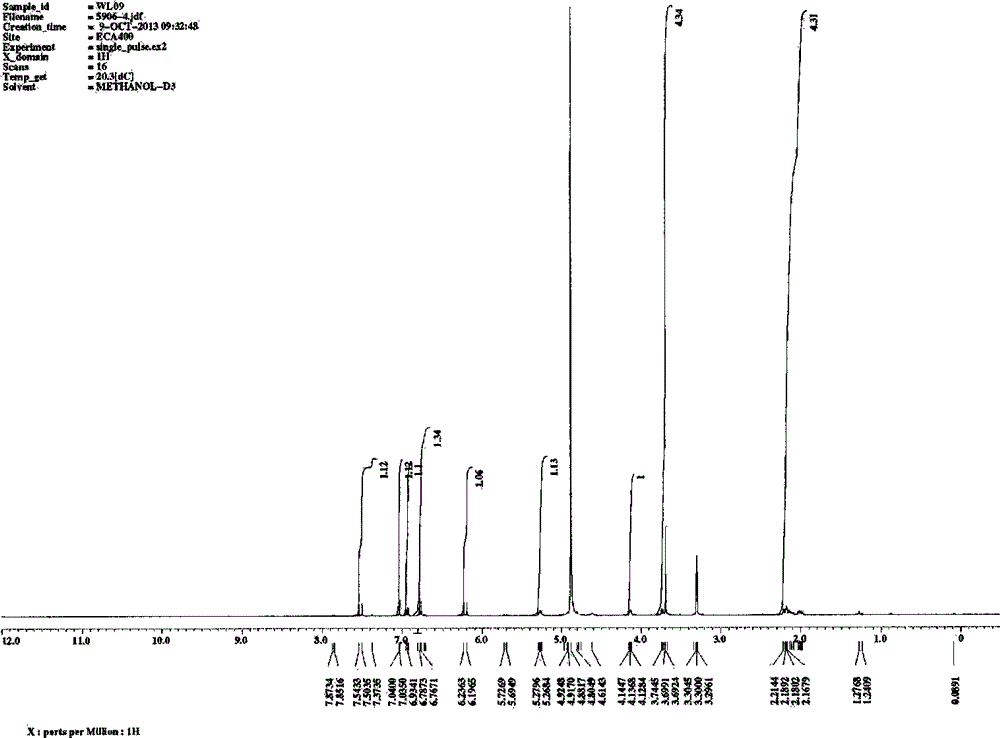
[0040] Implementation example 2: preparation and spectral analysis of each monomeric compound
[0041] Take 20kg of dried medicinal materials of P. chinensis, and use the method of Example 1 to prepare the elution site of 30% ethanol of P. spp. Take 30.7g of the obtained 30% ethanol elution fraction, dissolve it in 20ml water, filter and use reverse phase C 18 Silica gel column chromatography, gradient elution with different concentrations of aqueous methanol (10%, 20%, 30%, 40%, 50%, 70%, 95%), divided into sections F1-F46. Sections F19-20 are combined and recorded as component B, and then reversed phase C 18 Silica gel column chromatography, gradient elution with different concentrations of aqueous methanol (5%, 10%, 15%, 20%, 25%, 30%, 40%, 50%, 100%), divided into B1-B58. Where B8-B10 are combined, use LH-20 column chromatography, elute with methanol, and divide into B-8-1 to B-8-58, where components B-8-16 to B-8-26 are combined, and use silica gel Column chromatograph...
Embodiment 3
[0054] Implementation Example 3: Evaluation of the hemostatic effect of the total extract of Roropetus alba, different polar parts and monomeric compounds.
[0055] 1. The impact of the total extract of Example 1 on the bleeding time and bleeding amount by rat tail docking method
[0056] Experimental animals: Wistar rats, half male and half male, weighing 180g±10g, purchased from Beijing Weitong Lihua Company, and bred in the experimental animal center of the unit.
[0057] Test sample: the total extract of Example 1, dissolved with physiological saline.
[0058] Experimental method and results: 18 Wistar rats were randomly divided into 3 groups, 6 rats in each group. After the tail was docked 3cm from the root of the tail, intraperitoneal injection was administered, and the bleeding stop time and bleeding volume were observed; or intraperitoneal injection for 30 minutes Finally, the tail was docked at 3 cm from the root of the tail, and the bleeding stop time and bleeding v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com