Bilberry extract and preparation method thereof
An extract, English technology, applied in the field of European bilberry extract and the preparation of this extract, can solve problems such as difficult methods, and achieve the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The acidic aqueous solution containing bilberry total anthocyanins is prepared by one of the following methods.
[0075] Method A: Crack the lingonberry fruit into pulp with a tissue homogenizer. The volume fraction of 2-5 times the pulp mass is 30-80%, and the pH value is adjusted to acidic ethanol aqueous solution with hydrochloric acid, heated and refluxed at 40-60°C for 2-3 times, filtered, the filtrates are combined, the ethanol is recovered, concentrated .
[0076] Method B: Weighing a commercially available bilberry extract with a total anthocyanin content of 36% to prepare an aqueous solution.
Embodiment 2
[0078] The macroporous adsorption resin used is prepared by the following method:
[0079] Prepare an aqueous solution with a mass concentration of polyvinyl alcohol of 0.5-2% and a sodium chloride mass concentration of 5-10%, which is the aqueous phase of suspension polymerization; mix divinylbenzene (DVB) with a mass percentage of 50-80% and ester mono The body is mixed according to the feeding ratio, and the porogen and initiator are added, and after mixing evenly, it becomes the suspension polymerization oil phase. Wherein, the ester monomer is one or more of ethylene glycol dimethacrylate, methyl acrylate and methyl methacrylate. The feeding ratio of DVB and ester monomer is 19:1-7:3. The porogen can be one or more of toluene, 200# gasoline, cyclohexane, and methylcyclohexane, and the amount added is 1-3 times the total mass of DVB and ester monomers. The initiator is azobisisobutyronitrile or benzoyl peroxide, and the addition amount is 1-2% of the mass sum of DVB and ...
Embodiment 3
[0084] The macroporous adsorption resin prepared by the above method is fully swollen with ethanol, loaded into a resin column, washed with ethanol until the effluent is mixed with water in equal volumes and is not cloudy, and then washed with water until the effluent has no alcohol smell, and the macroporous adsorption resin column is obtained.
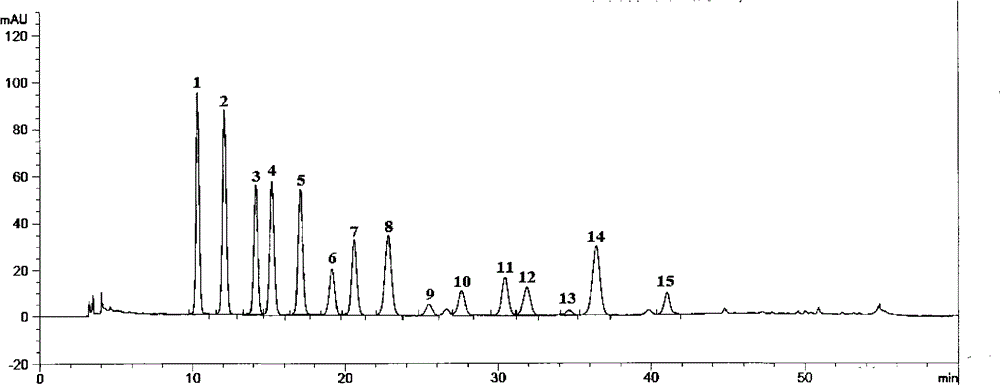
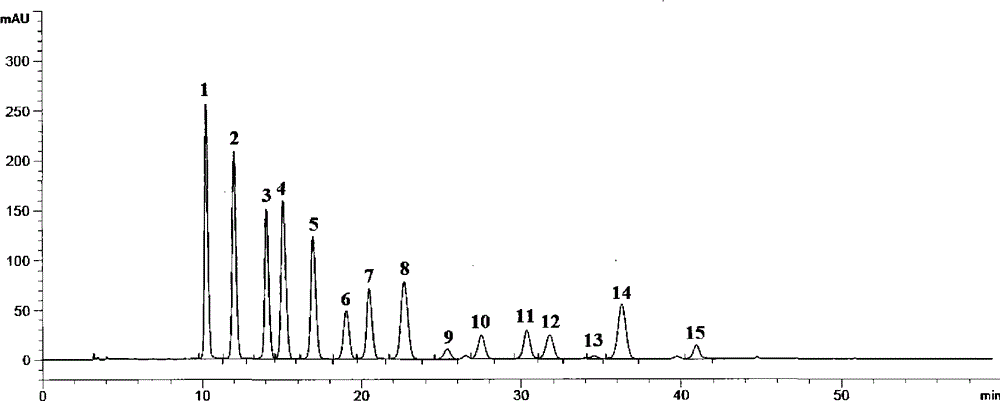
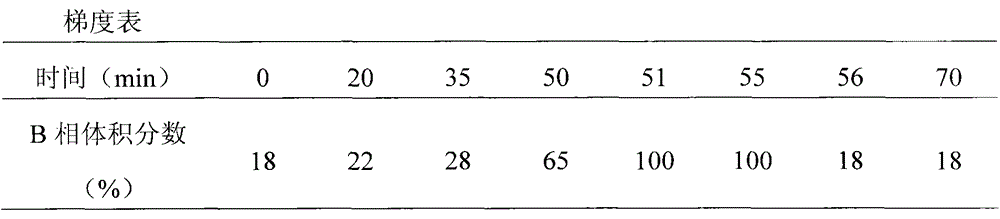
[0085] The acid aqueous solution is passed into the macroporous adsorption resin column obtained by the above method for adsorption. After the adsorption is completed, wash with acidic water until the effluent is clear, then use acidic ethanol or methanol or acetone aqueous solution as a desorbent for desorption, and receive the eluent in sections, preferably with a pH value between 2-5, and combine the total anthocyanins The part whose content is 55%-95%. Concentrate, spray dry, the air inlet temperature is 130-140°C, and the outlet air temperature is 60-65°C to obtain the finished product. The obtained results are listed in Table ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com