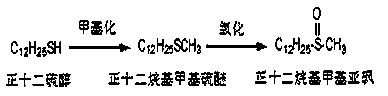
Method for preparing dodecyl methyl sulfoxide
A technology of n-dodecyl methyl sulfoxide and dodecyl methyl sulfide, applied in the field of n-dodecyl methyl sulfoxide preparation technology, can solve the problem of damage, unsuitability for industrialization, periodic acid Sodium oxidation is strong and other problems, and the oxidation selectivity is good, the reaction is easy to control, and the oxidation selectivity is high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
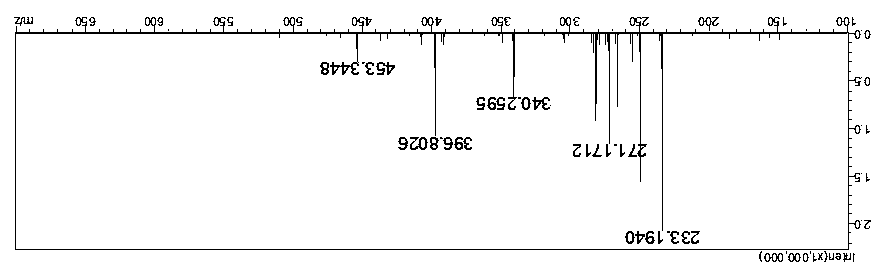
[0040] Reaction dosage: 40ml of dimethyl carbonate, 14g of potassium carbonate, 10g of dodecanethiol, 1 tablet of iodine, react in an oil bath at 95°C, and observe the reaction solution that has reacted for 8 hours by spotting the plate, which proves that the reaction has been completed.
[0041] After the reaction, filter with suction, rinse and dissolve with ethyl acetate, wash with saturated brine twice, then dry with anhydrous sodium sulfate, and concentrate by rotation (at a temperature of about 30 degrees) until no ethyl acetate drips out. The crude weight of the reaction solution is 3.35g, and 12.07g of hydrogen peroxide and 20ml of tetrahydrofuran are added in a ratio of 1:4 based on mass as the unit of conversion. The reaction temperature has been maintained at around 0-2 degrees. During this period, take a small amount of reaction solution in two test tubes, one is reacted with a small amount of sodium periodate, and the other is reacted with concentrated sulfuric aci...
Embodiment 2
[0044] Reaction dosage: 80ml of dimethyl carbonate, 28.02g of potassium carbonate, 20.12g of dodecyl mercaptan, 0.12g of potassium iodide, reacted in an oil bath at 95°C, and finally the reaction was basically completed by spotting plate observation. After processing and concentrating in the same way as the first batch, the sample solution was divided into three parts, as follows:
[0045] (1) React with 5.75g of reaction solution + 20ml of acetic acid + 20.72g of hydrogen peroxide, and a white solid appears after the reaction.
[0046](2) React 7.5g of reaction solution + 20ml of tetrahydrofuran + 27.03g of hydrogen peroxide + 0.5ml of concentrated sulfuric acid to react. After the reaction, let it stand still, and a needle-shaped white solid appears. Drying, the current preliminary weighing product is about 8g.
[0047] (3) React 4.01g of reaction solution + 20ml of tetrahydrofuran + 14.45g of hydrogen peroxide + 0.5g of phosphomolybdic acid for reaction. After the reaction...
Embodiment 3
[0049] Reaction dosage: 40ml of dimethyl carbonate, 21.02g of potassium carbonate, 10.24g of dodecyl mercaptan, 0.19g of potassium iodide, reacted in an oil bath at 95°C, and added 20ml of dimethyl carbonate and 9.98g of potassium carbonate after a period of reaction. For the reaction, after a period of reaction, the raw materials were still left by spotting plate observation, and 20ml of dimethyl carbonate was added for reaction, and finally the reaction was basically completed by spotting plate observation. According to the same method as in Example 1, after concentration and standing still, needle-shaped solids appeared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com