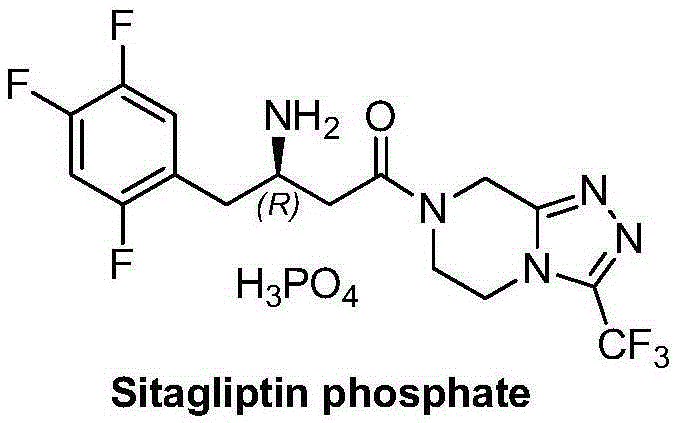
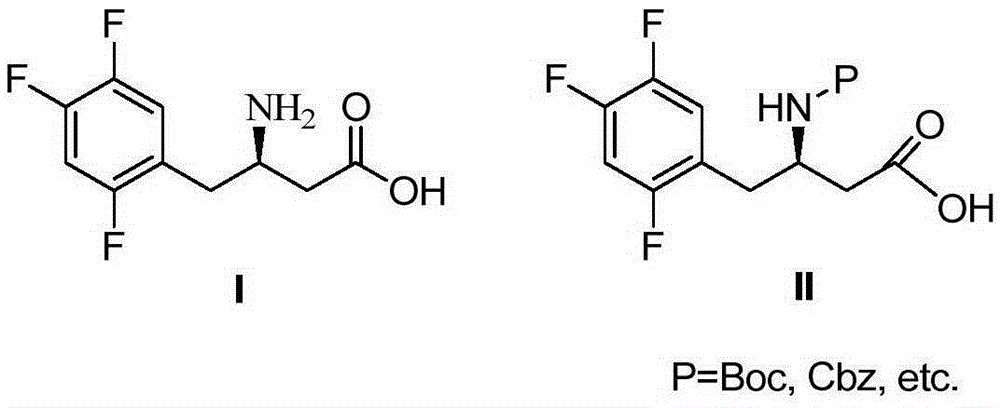
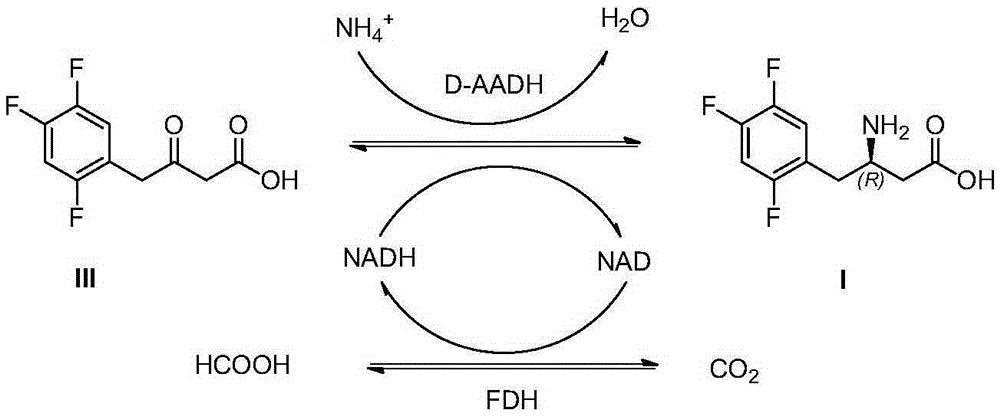
Sitagliptin and enzyme-chemical preparation method of intermediate of sitagliptin
A technology for enzymatic preparation and recycling of enzymes, which is applied in the field of medical biochemistry, can solve problems that have not been reported, and achieve the effects of reducing process routes, reducing production costs, and improving product purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 (R)-3-amino-4-(2,4,5-trifluorophenyl) butanoic acid (I)
[0033] Add 3-carbonyl-4-(2,4,5-trifluorophenyl)butanoic acid (18.6g) and NAD (0.1g) successively in potassium phosphate buffer (100ml, 0.1mol / l, pH=7.5) , ammonium formate (10g), dimethyl sulfoxide (10ml), formate dehydrogenase (5g), D-amino acid dehydrogenase (5g), stirred and reacted in a water bath at 30°C for 24h, when the conversion rate reached 95% as detected by HPLC , adjust the pH to 2-3 to terminate the reaction, filter to remove the precipitate, add an equal volume of ethyl acetate to the filtrate to extract 3 times, combine the extracts, add anhydrous sodium sulfate to dry, filter and concentrate to obtain (R)-3-amino- 4-(2,4,5-trifluorophenyl)butanoic acid (16.5g), the yield was 88%, and the ee value of the product was >99%.
Embodiment 2
[0034] Example 2 Preparation of (R)-N-tert-butoxycarbonyl-3-amino-4-(2,4,5-trifluorophenyl)butanoic acid (II)
[0035] Add (R)-3-amino-4-(2,4,5-trifluorophenyl) butanoic acid (10g) in the reaction flask, (Boc) 2 O (11.2g), sodium bicarbonate (7.9g), tetrahydrofuran (50ml) and water (50ml), react at room temperature for 24h, adjust the pH to 2-3, add ethyl acetate for extraction (50ml*3), and combine the extracts , added anhydrous sodium sulfate for drying treatment, filtered and concentrated to give (R)-N-tert-butoxycarbonyl-3-amino-4-(2,4,5-trifluorophenyl)butanoic acid (13.6g), yield 95%.
Embodiment 37-
[0036]Example 37-[(R)-3-(tert-butoxycarbonylamino)-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8- Preparation of tetrahydro-3-(trifluoromethyl)-1,2,4-triazol[4,3-a]pyrazole (V)
[0037] Add (R)-N-tert-butoxycarbonyl-3-amino-4-(2,4,5-trifluorophenyl)butanoic acid (10g) into the reaction flask, 3-(trifluoromethyl)-5 ,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride (12.1g), triethylamine (6.8g), 1-hydroxybenzo Triazole (6g) and 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (7.2g), tetrahydrofuran (80ml), reacted at room temperature for 12h, added water (80ml), The layers were separated, the aqueous layer was extracted with ethyl acetate (50ml*2), the organic layers were combined, dried by adding anhydrous sodium sulfate, concentrated by filtration, and crystallized from a mixed solvent of isopropanol / water (10:1, 50ml) to obtain 7-[ (R)-3-(tert-butoxycarbonylamino)-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3- (Trifluoromethyl)-1,2,4-tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com